200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 626-48-2
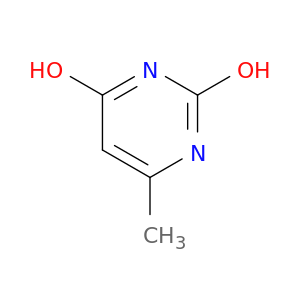
626-48-2 | 2,4-Dihydroxy-6-Methylpyrimidine
CAS No: 626-48-2 Catalog No: AG0032ZH MDL No:MFCD00006028
Product Description
Catalog Number:
AG0032ZH
Chemical Name:
2,4-Dihydroxy-6-Methylpyrimidine
CAS Number:
626-48-2
Molecular Formula:
C5H6N2O2
Molecular Weight:
126.1133
MDL Number:
MFCD00006028
IUPAC Name:
6-methyl-1H-pyrimidine-2,4-dione
InChI:
InChI=1S/C5H6N2O2/c1-3-2-4(8)7-5(9)6-3/h2H,1H3,(H2,6,7,8,9)
InChI Key:
SHVCSCWHWMSGTE-UHFFFAOYSA-N
SMILES:
Cc1cc(O)nc(n1)O
EC Number:
210-949-4
UNII:
5O052W0G6I
NSC Number:
9456
Properties
Complexity:
195
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
126.043g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
126.115g/mol
Monoisotopic Mass:
126.043g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
58.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.8
Literature
| Title | Journal |
|---|---|
| Effect of tissue-specific acetylcholinesterase inhibitor C-547 on α3β4 and αβεδ acetylcholine receptors in COS cells. | European journal of pharmacology 20120805 |
| [Stages of bone regeneration and foundation of pharmacоlogical correction of the mandible reparative osteogenesis]. | Stomatologiia 20120101 |
| Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. | Nature 20111208 |
| Synthesis of various 5-alkoxymethyluracil analogues and structure-cytotoxic activity relationship study. | Carbohydrate research 20111018 |
| Different sensitivities of rat skeletal muscles and brain to novel anti-cholinesterase agents, alkylammonium derivatives of 6-methyluracil (ADEMS). | British journal of pharmacology 20110601 |
| Hydrothermal synthesis, experimental and theoretical characterization of a novel cocrystal compound in the 2:1 stoichiometric ratio containing 6-methyluracil and dipicolinic acid. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20110501 |
| Identifying chelators for metalloprotein inhibitors using a fragment-based approach. | Journal of medicinal chemistry 20110127 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| 1,3-Bis(4-meth-oxy-benz-yl)-6-methyl-pyrimidine-2,4(1H,3H)-dione. | Acta crystallographica. Section E, Structure reports online 20100701 |
| An evaluation of the chick cardiomyocyte micromass system for identification of teratogens in a blind trial. | Reproductive toxicology (Elmsford, N.Y.) 20091201 |
| Mechanisms of cardiac muscle insensitivity to a novel acetylcholinesterase inhibitor C-547. | Journal of cardiovascular pharmacology 20090201 |
| Different sensitivity of miniature endplate currents in rat external and internal intercostal muscles to the acetylcholinesterase inhibitor C-547 as compared with diaphragm and extensor digitorum longus. | Physiological research 20090101 |
| Compounds with the dioxopyrimidine cycle inhibit cholinesterases from different groups of animals. | Chemico-biological interactions 20080925 |
| Tetrakis- and tris(1-Methyluracil) complexes of Pt(II): formation and properties of a carbon-bonded nucleobase species as well as of heternonuclear derivatives. | Inorganic chemistry 20071224 |
| The design and synthesis of N-1-alkylated-5-aminoarylalkylsubstituted-6-methyluracils as potential non-nucleoside HIV-1 RT inhibitors. | Bioorganic & medicinal chemistry 20071201 |
| Selective oxidation of key functional groups in cyanotoxins during drinking water ozonation. | Environmental science & technology 20070615 |
| Effect of a tetraalkylammonium derivative of 6-methyluracil from a new class of acetylcholinesterase inhibitors on the endplate potential amplitude in muscles of different function types under high-frequency nerve stimulation. | Doklady biological sciences : proceedings of the Academy of Sciences of the USSR, Biological sciences sections 20070101 |
| [Studying the nootropic effects of betamecil]. | Eksperimental'naia i klinicheskaia farmakologiia 20070101 |
| [Realization of optic activatory mechanism in laser-medication impact]. | Voprosy kurortologii, fizioterapii, i lechebnoi fizicheskoi kultury 20060101 |
| Different sensitivity of miniature endplate currents of the rat extensor digitorum longus, soleus and diaphragm muscles to a novel acetylcholinesterase inhibitor C-547. | Physiological research 20060101 |
| Reaction of substituted pyrimidines with photochemically generated t-BuO* radicals. | Indian journal of biochemistry & biophysics 20051201 |
| Atropisomeric property of 1-(2,6-difluorobenzyl)-3-[(2R)-amino-2-phenethyl]-5-(2-fluoro-3-methoxyphenyl)-6-methyluracil. | Chirality 20051101 |
| Electron impact mass spectral study of 1,2-di-o-(m- and p-)nitro-(bromo-)benzyl-2-thio-6-methyluracils and 1,2-di-o-(m- and p-)nitro-(bromo-)benzyl-2-thio-5-bromo-6-methyluracils. | Rapid communications in mass spectrometry : RCM 20050101 |
| Synthesis and structure-activity relationships of (R)-1-alkyl-3-[2-(2-amino)phenethyl]-5-(2-fluorophenyl)-6-methyluracils as human GnRH receptor antagonists. | Bioorganic & medicinal chemistry letters 20040503 |
| Effect of tetraalkylammonium derivatives of 6-methyluracil on the endplate potentials of muscles of different functional types. | Doklady biological sciences : proceedings of the Academy of Sciences of the USSR, Biological sciences sections 20040101 |
| Antiparasitic activity of highly conjugated pyrimidine-2,4-dione derivatives. | Farmaco (Societa chimica italiana : 1989) 20031201 |
| Synthesis and structure-activity relationships of 1-arylmethyl-3-(2-aminopropyl)-5-aryl-6-methyluracils as potent GnRH receptor antagonists. | Bioorganic & medicinal chemistry letters 20031006 |
| Synthesis and structure-activity relationships of 1-arylmethyl-3-(1-methyl-2-amino)ethyl-5-aryl-6-methyluracils as antagonists of the human GnRH Receptor. | Bioorganic & medicinal chemistry letters 20031006 |
| [Current methods for local drug therapy of infected wounds]. | Khirurgiia 20030101 |
| Pyrrolidino-DNA. | Nucleosides, nucleotides & nucleic acids 20030101 |
| Biological activity of bacterial lectins and their molecular complexes with heterocyclic bis-adducts. | Mikrobiolohichnyi zhurnal (Kiev, Ukraine : 1993) 20030101 |
| The acidity of uracil and uracil analogs in the gas phase: four surprisingly acidic sites and biological implications. | Journal of the American Society for Mass Spectrometry 20020801 |
| Genotoxicity study of a new tetraalkylammonium derivative of 6-methyluracil (agent No. 547). | Archives of toxicology 20020301 |
| [Photophoresis of methyluracil ointment in the complex treatment of temporomandibular pain syndrome]. | Voprosy kurortologii, fizioterapii, i lechebnoi fizicheskoi kultury 20020101 |
| [Studies of biological activity of mineral oil dialkyl disulfide (an experimental study)]. | Meditsina truda i promyshlennaia ekologiia 20020101 |
| [Morphological aspects of dermatotrophic action of methyluracil applied epicutaneously]. | Morfologiia (Saint Petersburg, Russia) 20020101 |
| [Surgical infected wound treatment in patients with severe gastrointestinal ulcer hemorrhage]. | Klinichna khirurhiia 20020101 |
| Effect of tetraalkylammonium derivative of 6-methyluracil on amplitude and temporal parameters of miniature endplate potentials in frog neuromuscular junction. | Bulletin of experimental biology and medicine 20010501 |
| Tetraalkylammonium derivatives of 6-methyluracil, a new class of cholinesterase inhibitors: characteristics of interaction with cholinesterases from different groups of animals. | Doklady. Biochemistry and biophysics 20010101 |
| Structure-activity relationship of ligands of uracil phosphoribosyltransferase from Toxoplasma gondii. | Biochemical pharmacology 19940817 |
Related Products
© 2019 Angene International Limited. All rights Reserved.