200,000+ products from a single source!
sales@angenechem.com
Home > Boronic Acids > 4363-34-2
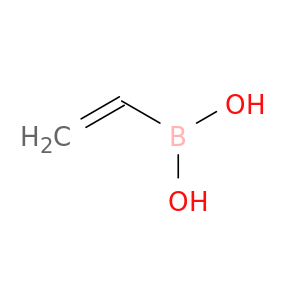
4363-34-2 | Boronic acid, ethenyl-
CAS No: 4363-34-2 Catalog No: AG00I8CB MDL No:MFCD02258951
Product Description
Catalog Number:
AG00I8CB
Chemical Name:
Boronic acid, ethenyl-
CAS Number:
4363-34-2
Molecular Formula:
C2H5BO2
Molecular Weight:
71.8709
MDL Number:
MFCD02258951
IUPAC Name:
ethenylboronic acid
InChI:
InChI=1S/C2H5BO2/c1-2-3(4)5/h2,4-5H,1H2
InChI Key:
YFXCNIVBAVFOBX-UHFFFAOYSA-N
SMILES:
OB(C=C)O
Properties
Complexity:
34.6
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
72.038g/mol
Formal Charge:
0
Heavy Atom Count:
5
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
71.87g/mol
Monoisotopic Mass:
72.038g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
40.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Lewis acid promoted highly diastereoselective Petasis Borono-Mannich reaction: efficient synthesis of optically active β,γ-unsaturated α-amino acids. | Organic letters 20120420 |
| Highly selective methods for synthesis of internal (α-) vinylboronates through efficient NHC-Cu-catalyzed hydroboration of terminal alkynes. Utility in chemical synthesis and mechanistic basis for selectivity. | Journal of the American Chemical Society 20110525 |
| Total synthesis of fostriecin: via a regio- and stereoselective polyene hydration, oxidation, and hydroboration sequence. | Organic letters 20100903 |
| Vicinal diboronates in high enantiomeric purity through tandem site-selective NHC-Cu-catalyzed boron-copper additions to terminal alkynes. | Journal of the American Chemical Society 20091230 |
| Diastereoselective addition of zincated hydrazones to alkenylboronates and stereospecific trapping of boron/zinc bimetallic intermediates by carbon electrophiles. | Journal of the American Chemical Society 20081119 |
| Synthesis of amphidinolide E C10-C26 fragment. | Organic letters 20081106 |
| Synthesis and evaluation of the cytotoxicity of apoptolidinones A and D. | The Journal of organic chemistry 20080704 |
| A new catalytic route to boryl- and borylsilyl-substituted buta-1,3-dienes. | Chemistry (Weinheim an der Bergstrasse, Germany) 20080101 |
| Sequential Pd-catalyzed asymmetric allene diboration/alpha-aminoallylation. | Journal of the American Chemical Society 20060111 |
| Asymmetric total synthesis of (-)-spirofungin A and (+)-spirofungin B. | Organic letters 20051208 |
| Boron-directed regio- and stereoselective enyne cross metathesis: efficient synthesis of vinyl boronate containing 1,3-dienes. | Organic letters 20050428 |
| Synthesis of beta,beta-disubstituted vinyl boronates via the ruthenium-catalyzed Alder ene reaction of borylated alkynes and alkenes. | Journal of the American Chemical Society 20050316 |
| Efficient synthesis of aryl vinyl ethers exploiting 2,4,6-trivinylcyclotriboroxane as a vinylboronic acid equivalent. | The Journal of organic chemistry 20040723 |
| Total synthesis of (-)-bafilomycin A(1). | Journal of the American Chemical Society 20020619 |
| Total synthesis of rutamycin B, a macrolide antibiotic from Streptomyces aureofaciens. | The Journal of organic chemistry 20010727 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







