200,000+ products from a single source!
sales@angenechem.com
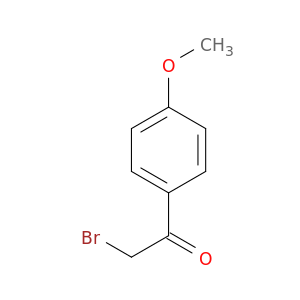
2632-13-5 | 2-Bromo-1-(4-methoxyphenyl)ethanone
CAS No: 2632-13-5 Catalog No: AG00335F MDL No:MFCD00000201
Product Description
Catalog Number:
AG00335F
Chemical Name:
2-Bromo-1-(4-methoxyphenyl)ethanone
CAS Number:
2632-13-5
Molecular Formula:
C9H9BrO2
Molecular Weight:
229.0706
MDL Number:
MFCD00000201
IUPAC Name:
2-bromo-1-(4-methoxyphenyl)ethanone
InChI:
InChI=1S/C9H9BrO2/c1-12-8-4-2-7(3-5-8)9(11)6-10/h2-5H,6H2,1H3
InChI Key:
XQJAHBHCLXUGEP-UHFFFAOYSA-N
SMILES:
BrCC(=O)c1ccc(cc1)OC
EC Number:
220-118-8
NSC Number:
129010
Properties
Complexity:
151
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
227.979g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
229.073g/mol
Monoisotopic Mass:
227.979g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
26.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.5
Literature
| Title | Journal |
|---|---|
| 6-Fluoro-2-(4-meth-oxy-phen-yl)imidazo[2,1-b][1,3]benzothia-zole. | Acta crystallographica. Section E, Structure reports online 20111201 |
| Switching reversibility to irreversibility in glycogen synthase kinase 3 inhibitors: clues for specific design of new compounds. | Journal of medicinal chemistry 20110623 |
| 2-Isobutyl-6-(4-meth-oxy-phen-yl)imidazo[2,1-b][1,3,4]thia-diazole. | Acta crystallographica. Section E, Structure reports online 20110201 |
| Effects of a fluorescent Myosin light chain phosphatase inhibitor on prostate cancer cells. | Frontiers in oncology 20110101 |
| 2-Bromo-1-(4-methoxy-phen-yl)ethanone. | Acta crystallographica. Section E, Structure reports online 20090901 |
| Thienyl and phenyl alpha-halomethyl ketones: new inhibitors of glycogen synthase kinase (GSK-3beta) from a library of compound searching. | Journal of medicinal chemistry 20031023 |
| alpha-bromoacetophenone derivatives as neutral protein tyrosine phosphatase inhibitors: structure-Activity relationship. | Bioorganic & medicinal chemistry letters 20021104 |
Related Products
© 2019 Angene International Limited. All rights Reserved.