200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 125279-72-3
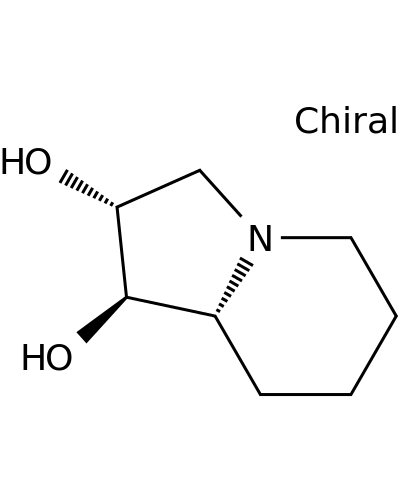
125279-72-3 | 1,2-Indolizinediol, octahydro-, (1R,2R,8aR)-
CAS No: 125279-72-3 Catalog No: AG000NFM MDL No:
Product Description
Catalog Number:
AG000NFM
Chemical Name:
1,2-Indolizinediol, octahydro-, (1R,2R,8aR)-
CAS Number:
125279-72-3
Molecular Formula:
C8H15NO2
Molecular Weight:
157.2102
IUPAC Name:
(1R,2R,8aR)-1,2,3,5,6,7,8,8a-octahydroindolizine-1,2-diol
InChI:
InChI=1S/C8H15NO2/c10-7-5-9-4-2-1-3-6(9)8(7)11/h6-8,10-11H,1-5H2/t6-,7-,8-/m1/s1
InChI Key:
SQECYPINZNWUTE-BWZBUEFSSA-N
SMILES:
O[C@@H]1CN2[C@@H]([C@H]1O)CCCC2
Properties
Complexity:
151
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
157.11g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
157.213g/mol
Monoisotopic Mass:
157.11g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
43.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.3
Literature
| Title | Journal |
|---|---|
| Synthesis of 1,2-dihydroxyindolizidines from 1-(2-pyridyl)-2-propen-1-ol. | The Journal of organic chemistry 20111118 |
| The novel proapoptotic activity of nonnatural enantiomer of Lentiginosine. | Glycobiology 20100501 |
| Sterically biased 3,3-sigmatropic rearrangement of chiral allylic azides: application to the total syntheses of alkaloids. | The Journal of organic chemistry 20080815 |
| Binding of sulfonium-ion analogues of di-epi-swainsonine and 8-epi-lentiginosine to Drosophila Golgi alpha-mannosidase II: the role of water in inhibitor binding. | Proteins 20080515 |
| Efficient synthesis of (+)-1,8,8a-tri-epi-swainsonine, (+)-1,2-di-epi-lentiginosine, (+)-9a-epi-homocastanospermine and (-)-9-deoxy-9a-epi-homocastanospermine from a D-glucose-derived aziridine carboxylate, and study of their glycosidase inhibitory activities. | Organic & biomolecular chemistry 20080221 |
| A concise synthesis of lentiginosine derivatives using a pyridinium formation via the Mitsunobu reaction. | The Journal of organic chemistry 20080201 |
| New access to indolizidine and pyrrolizidine alkaloids from an enantiopure proline: total syntheses of (-)-lentiginosine and (1R,2R,7aR)-dihydroxypyrrolizidine. | The Journal of organic chemistry 20070720 |
| Regioselective and diastereoselective amination of polybenzyl ethers using chlorosulfonyl isocyanate: total syntheses of 1,4-dideoxy-1,4-imino-D-arabinitol and (-)-lentiginosine. | Organic letters 20060831 |
| Asymmetric syntheses of (-)-lentiginosine and an original pyrrolizidinic analogue thereof from a versatile epoxyamine intermediate. | Organic & biomolecular chemistry 20050721 |
| Stereoselective allyl amine synthesis through enantioselective addition of diethylzinc and [1,3]-chirality transfer: synthesis of lentiginosine and polyoxamic acid derivative. | Chemistry (Weinheim an der Bergstrasse, Germany) 20050304 |
| Concise asymmetric syntheses of (-)-lentiginosine and of its pyrrolizidinic analogue. | Chemical communications (Cambridge, England) 20030307 |
| Total synthesis of (-)- and (+)-lentiginosine. | The Journal of organic chemistry 20020628 |
| Synthesis of polyhydroxyindolizidines from 5,6-dihydro-2H-pyran-2-one. | Carbohydrate research 20011207 |
| Enantiocontrolled preparation of indolizidines: synthesis of (-)-2-epilentiginosine and (+)-lentiginosine. | The Journal of organic chemistry 20010810 |
Related Products
© 2019 Angene International Limited. All rights Reserved.