200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 239-09-8
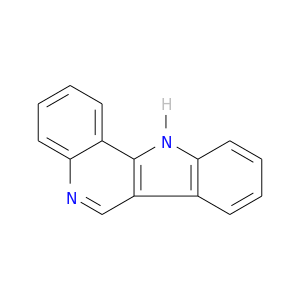
239-09-8 | 11H-Indolo[3,2-c]quinoline
CAS No: 239-09-8 Catalog No: AG006XTE MDL No:
Product Description
Catalog Number:
AG006XTE
Chemical Name:
11H-Indolo[3,2-c]quinoline
CAS Number:
239-09-8
Molecular Formula:
C15H10N2
Molecular Weight:
218.2533
IUPAC Name:
11H-indolo[3,2-c]quinoline
InChI:
InChI=1S/C15H10N2/c1-4-8-14-10(5-1)12-9-16-13-7-3-2-6-11(13)15(12)17-14/h1-9,17H
InChI Key:
LQRUVABVXQZQMV-UHFFFAOYSA-N
SMILES:
c1ccc2c(c1)c1[nH]c3c(c1cn2)cccc3
Properties
Complexity:
291
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
218.084g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
218.259g/mol
Monoisotopic Mass:
218.084g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
28.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.7
Literature
| Title | Journal |
|---|---|
| An alternative one-pot gold-catalyzed approach to the assembly of 11H-indolo[3,2-c]quinolines. | Organic & biomolecular chemistry 20121014 |
| Structure-activity relationships of highly cytotoxic copper(II) complexes with modified indolo[3,2-c]quinoline ligands. | Inorganic chemistry 20101206 |
| Synthesis and antiproliferative evaluation of certain indolo[3,2-c]quinoline derivatives. | Bioorganic & medicinal chemistry 20100301 |
| Design of antineoplastic agents based on the '2-phenylnaphthalene-type' structural pattern--synthesis and biological activity studies of 11H-indolo[3.2-c]quinoline derivatives. | European journal of medicinal chemistry 20030101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.