200,000+ products from a single source!
sales@angenechem.com
Home > Nitro Compounds > 2127-10-8
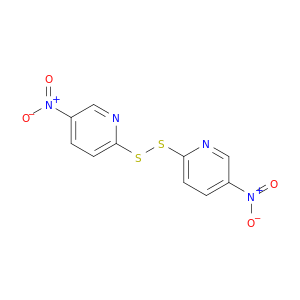
2127-10-8 | 1,2-Bis(5-nitropyridin-2-yl)disulfane
CAS No: 2127-10-8 Catalog No: AG003DC9 MDL No:MFCD00006453
Product Description
Catalog Number:
AG003DC9
Chemical Name:
1,2-Bis(5-nitropyridin-2-yl)disulfane
CAS Number:
2127-10-8
Molecular Formula:
C10H6N4O4S2
Molecular Weight:
310.3090
MDL Number:
MFCD00006453
IUPAC Name:
5-nitro-2-[(5-nitropyridin-2-yl)disulfanyl]pyridine
InChI:
InChI=1S/C10H6N4O4S2/c15-13(16)7-1-3-9(11-5-7)19-20-10-4-2-8(6-12-10)14(17)18/h1-6H
InChI Key:
ROUFCTKIILEETD-UHFFFAOYSA-N
SMILES:
[O-][N+](=O)c1ccc(nc1)SSc1ccc(cn1)[N+](=O)[O-]
EC Number:
218-344-7
Properties
Complexity:
328
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
309.983g/mol
Formal Charge:
0
Heavy Atom Count:
20
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
310.302g/mol
Monoisotopic Mass:
309.983g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
168A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.2
Literature
| Title | Journal |
|---|---|
| The use of 2,2'-dithiobis(5-nitropyridine) (DTNP) for deprotection and diselenide formation in protected selenocysteine-containing peptides. | Journal of peptide science : an official publication of the European Peptide Society 20120301 |
| 2,2'-Dithiobis(5-nitropyridine) (DTNP) as an effective and gentle deprotectant for common cysteine protecting groups. | Journal of peptide science : an official publication of the European Peptide Society 20120101 |
| Redox modulation of A-type K+ currents in pain-sensing dorsal root ganglion neurons. | Biochemical and biophysical research communications 20080606 |
| Studies on deprotection of cysteine and selenocysteine side-chain protecting groups. | Journal of peptide science : an official publication of the European Peptide Society 20070201 |
| Nitric oxide activates TRP channels by cysteine S-nitrosylation. | Nature chemical biology 20061101 |
| Disinactivation of N-type inactivation of voltage-gated K channels by an erbstatin analogue. | The Journal of biological chemistry 20040709 |
| Inactivation of the human papillomavirus-16 E6 oncoprotein by organic disulfides. | Bioorganic & medicinal chemistry 20001101 |
| Evaluation of selected chemotypes in coupled cellular and molecular target-based screens identifies novel HIV-1 zinc finger inhibitors. | Journal of medicinal chemistry 19960913 |
Related Products
© 2019 Angene International Limited. All rights Reserved.