200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 13177-38-3
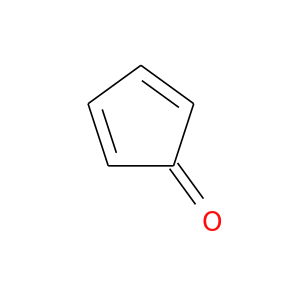
13177-38-3 | 2,4-Cyclopentadien-1-one
CAS No: 13177-38-3 Catalog No: AG000ZPN MDL No:
Product Description
Catalog Number:
AG000ZPN
Chemical Name:
2,4-Cyclopentadien-1-one
CAS Number:
13177-38-3
Molecular Formula:
C5H4O
Molecular Weight:
80.0847
IUPAC Name:
cyclopenta-2,4-dien-1-one
InChI:
InChI=1S/C5H4O/c6-5-3-1-2-4-5/h1-4H
InChI Key:
FQQOMPOPYZIROF-UHFFFAOYSA-N
SMILES:
O=C1C=CC=C1
Properties
Complexity:
106
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
80.026g/mol
Formal Charge:
0
Heavy Atom Count:
6
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
80.086g/mol
Monoisotopic Mass:
80.026g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
17.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.8
Literature
| Title | Journal |
|---|---|
| 4,5-Dihydro-cyclo-penta-[b]thio-phen-6-one. | Acta crystallographica. Section E, Structure reports online 20120201 |
| Thermal decomposition mechanisms of the methoxyphenols: formation of phenol, cyclopentadienone, vinylacetylene, and acetylene. | The journal of physical chemistry. A 20111124 |
| Pharmacological targets in the ubiquitin system offer new ways of treating cancer, neurodegenerative disorders and infectious diseases. | Expert reviews in molecular medicine 20111101 |
| Cnidarians as a source of new marine bioactive compounds--an overview of the last decade and future steps for bioprospecting. | Marine drugs 20110101 |
| Synthesis and structural analysis of a novel iodinated cyclopentadienone via ring-contraction iodination and its application in synthesis of alkyne-functionalized cyclopentadienones. | Chemical communications (Cambridge, England) 20101121 |
| syn-Dispiro-[1,3-dioxolane-2,17'-penta-cyclo-[12.2.1.1.0.0]octa-decane-18',2''-[1,3]dioxolane]-7',15'-diene. | Acta crystallographica. Section E, Structure reports online 20101101 |
| anti-1',6',7',8',9',14',15',16'-Octa-chloro-dispiro-[1,3-dioxolane-2,17'-penta-cyclo-[12.2.1.1.0.0]octa-decane-18',2''-1,3-dioxolane]-7',15'-diene. | Acta crystallographica. Section E, Structure reports online 20100801 |
| Synthesis of [RhCl(CO)(cyclopentadienone)]2 from [RhCl(cod)]2 and a 1,6-diyne under CO: application to Rh(I)-catalyzed tandem [2+2+1] carbonylative cycloaddition of diynes and Claisen rearrangement. | Chemical communications (Cambridge, England) 20100521 |
| Energy barriers of vinylidene carbene reactions from the anti-hermitian contracted Schrödinger equation. | The journal of physical chemistry. A 20100114 |
| Crystallographic evidence for cyclopropane ring formation in isotwistenes obtained by thermal cascade reactions of cyclopentadienone with acyclic conjugated dienes. | Chemical & pharmaceutical bulletin 20090701 |
| anti-Tricyclo-[4.2.1.1]deca-3,7-diene-9,10-dione. | Acta crystallographica. Section E, Structure reports online 20090401 |
| Cyclopentadienone iron alcohol complexes: synthesis, reactivity, and implications for the mechanism of iron-catalyzed hydrogenation of aldehydes. | Journal of the American Chemical Society 20090225 |
| Measuring aromaticity with the dimethyldihydropyrene ring current probe. Experimental and computational studies of the fulvenes and the strongly antiaromatic cyclopentadienone reveal large Mills-Nixon-type bond localization effects. Synthesis of fulvene-fused dihydropyrenes. | Journal of the American Chemical Society 20090114 |
| Organic semiconducting materials from sulfur-hetero benzo[k]fluoranthene derivatives: synthesis, photophysical properties, and thin film transistor fabrication. | The Journal of organic chemistry 20080718 |
| Synthesis, structure, and reactivity of naphthalyne-Co2(CO)6 complexes. | Journal of the American Chemical Society 20080521 |
| Predicting the UV-vis spectra of Tetraarylcyclopentadienones: Using DFT molecular orbital energies to model electronic transitions of organic materials. | The Journal of organic chemistry 20080418 |
| Multichromophoric polyphenylene dendrimers: toward brilliant light emitters with an increased number of fluorophores. | Journal of the American Chemical Society 20070418 |
| 2-substituted benzofuran fragment ion formation in the electron ionization mass spectra of 6-alkyl- and 6-aryldibenzo(d,f)(1,3)dioxepine derivatives. 1. Spirocyclization of the molecular ions in the gas phase. | Rapid communications in mass spectrometry : RCM 20070101 |
| Intramolecular reactions in pseudo-geminally substituted [2.2]paracyclophanes. | Chemistry (Weinheim an der Bergstrasse, Germany) 20070101 |
| Cyclopentadienone synthesis by rhodium(I)-catalyzed [3 + 2] cycloaddition reactions of cyclopropenones and alkynes. | Journal of the American Chemical Society 20061122 |
| Hydroxyl-directed conjugate additions of carbon nucleophiles to cyclopentadienones. | Organic letters 20060720 |
| Measuring antiaromaticity by an analysis of ring current and coupling constant changes in a cyclopentadienone-fused dihydropyrene. | Journal of the American Chemical Society 20051123 |
| 1,1-Cycloaddition of oxalyl dichloride with dialkenylmetal compounds: formation of cyclopentadienone derivatives by the reaction of 1,4,-dilithio-1,3-dienes or zirconacyclopentadienes with oxalyl chloride in the presence of CuCl. | Journal of the American Chemical Society 20050608 |
| Decomposition of neutral, singly and doubly protonated benzoquinone in the gas phase. | Chemistry (Weinheim an der Bergstrasse, Germany) 20050107 |
| PuPHOS: a synthetically useful chiral bidentate ligand for the intermolecular Pauson-Khand reaction. | The Journal of organic chemistry 20041112 |
| 2,5-Diphenyl-3,4-bis(2-pyridyl)cyclopenta-2,4-dien-1-one as a redox-active chelating ligand. | Chemistry (Weinheim an der Bergstrasse, Germany) 20041105 |
| Thermal and palladium-catalyzed [3 + 2] synthesis of cyclopentadienone acetals from cyclopropenone acetals and acetylenes. | Organic letters 20040930 |
| Punaglandins, chlorinated prostaglandins, function as potent Michael receptors to inhibit ubiquitin isopeptidase activity. | Journal of medicinal chemistry 20040408 |
| Mechanism of ruthenium-catalyzed hydrogen transfer reactions. Concerted transfer of OH and CH hydrogens from an alcohol to a (Cyclopentadienone)ruthenium complex. | The Journal of organic chemistry 20031003 |
| A bispericyclic transition structure allows for efficient relief of antiaromaticity enhancing reactivity and endo stereoselectivity in the dimerization of the fleeting cyclopentadienone. | The Journal of organic chemistry 20030725 |
| In situ generation of endohedral metallocenophanes of cobalt and rhodium with one cyclopentadienone unit. | Organic letters 20020822 |
| Stereoselective synthesis of (E)-4-alkylidenecyclopent-2-en-1-ones by a tandem ring closure-Michael addition-elimination. | Organic letters 20010503 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







