200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 113826-06-5
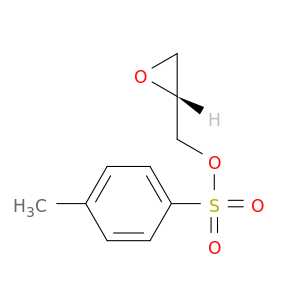
113826-06-5 | 2-Oxiranemethanol, 2-(4-methylbenzenesulfonate), (2R)-
CAS No: 113826-06-5 Catalog No: AG0009TL MDL No:MFCD00010834
Product Description
Catalog Number:
AG0009TL
Chemical Name:
2-Oxiranemethanol, 2-(4-methylbenzenesulfonate), (2R)-
CAS Number:
113826-06-5
Molecular Formula:
C10H12O4S
Molecular Weight:
228.2649
MDL Number:
MFCD00010834
IUPAC Name:
[(2R)-oxiran-2-yl]methyl 4-methylbenzenesulfonate
InChI:
InChI=1S/C10H12O4S/c1-8-2-4-10(5-3-8)15(11,12)14-7-9-6-13-9/h2-5,9H,6-7H2,1H3/t9-/m1/s1
InChI Key:
NOQXXYIGRPAZJC-SECBINFHSA-N
SMILES:
Cc1ccc(cc1)S(=O)(=O)OC[C@@H]1OC1
UNII:
62V982IWII
Properties
Complexity:
298
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
228.046g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
228.262g/mol
Monoisotopic Mass:
228.046g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
64.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.1
Literature
| Title | Journal |
|---|---|
| Enantiomeric separation of glycidyl tosylate by CE: application to the study of catalytic asymmetric epoxidation of allyl alcohol. | Electrophoresis 20081101 |
| Development and validation of a chiral HPLC method for rapid screening of allylic alcohol asymmetric epoxidation processes. | Analytica chimica acta 20080616 |
| Ru-catalyzed alkene-alkyne coupling. Total synthesis of amphidinolide P. | Journal of the American Chemical Society 20051221 |
| Synthesis of calix[4]arene and porphyrin tethering four chiral five-membered cyclic carbonates. | Enantiomer 20020101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.