200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1138-52-9
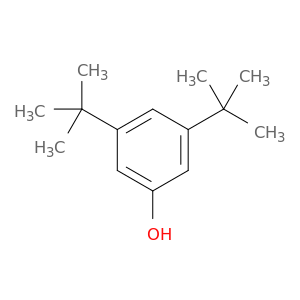
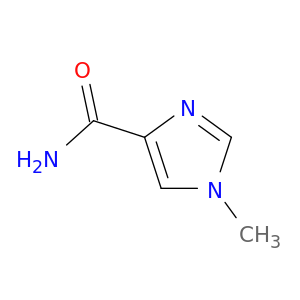
1138-52-9 | Phenol, 3,5-bis(1,1-dimethylethyl)-
CAS No: 1138-52-9 Catalog No: AG0009PL MDL No:MFCD00008829
Product Description
Catalog Number:
AG0009PL
Chemical Name:
Phenol, 3,5-bis(1,1-dimethylethyl)-
CAS Number:
1138-52-9
Molecular Formula:
C14H22O
Molecular Weight:
206.3239
MDL Number:
MFCD00008829
IUPAC Name:
3,5-ditert-butylphenol
InChI:
InChI=1S/C14H22O/c1-13(2,3)10-7-11(14(4,5)6)9-12(15)8-10/h7-9,15H,1-6H3
InChI Key:
ZDWSNKPLZUXBPE-UHFFFAOYSA-N
SMILES:
Oc1cc(cc(c1)C(C)(C)C)C(C)(C)C
EC Number:
214-513-4
UNII:
F4SMH8G9W7
NSC Number:
68209
Properties
Complexity:
184
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
206.167g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
206.329g/mol
Monoisotopic Mass:
206.167g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
20.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.9
Literature
| Title | Journal |
|---|---|
| Combining the benefits of homogeneous and heterogeneous catalysis with tunable solvents and nearcritical water. | Molecules (Basel, Switzerland) 20101116 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Combining homogeneous catalysis with heterogeneous separation using tunable solvent systems. | The journal of physical chemistry. A 20100325 |
| Photoinduced energy transfer processes within dyads of metallophthalocyanines compactly fused to a ruthenium(II) polypyridine chromophore. | The Journal of organic chemistry 20070928 |
| Models for the molybdenum hydroxylases: synthesis, characterization and reactivity of cis-oxosulfido-Mo(VI) complexes. | Journal of the American Chemical Society 20060111 |
| Dye-sensitized photooxygenation of the C=N bond. 5. substituent effects on the cleavage of the C=N bond of C-aryl-N-aryl-N-methylhydrazones. | The Journal of organic chemistry 20050527 |
| 4D-QSAR analysis of a set of propofol analogues: mapping binding sites for an anesthetic phenol on the GABA(A) receptor. | Journal of medicinal chemistry 20020718 |
| [Determination of 2,4,6-tri-tert-butylphenol and related compounds in foods]. | Shokuhin eiseigaku zasshi. Journal of the Food Hygienic Society of Japan 20011201 |
| Autoxidation of substituted phenols catalyzed by cobalt Schiff base complexes in supercritical carbon dioxide. | Inorganic chemistry 20010702 |
Related Products
© 2019 Angene International Limited. All rights Reserved.