200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1022-45-3
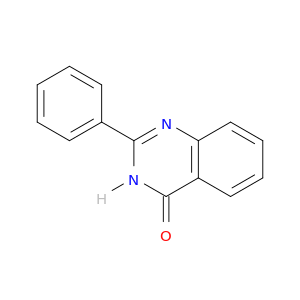
1022-45-3 | 4(3H)-Quinazolinone, 2-phenyl-
CAS No: 1022-45-3 Catalog No: AG0006W4 MDL No:MFCD00451430
Product Description
Catalog Number:
AG0006W4
Chemical Name:
4(3H)-Quinazolinone, 2-phenyl-
CAS Number:
1022-45-3
Molecular Formula:
C14H10N2O
Molecular Weight:
222.2420
MDL Number:
MFCD00451430
IUPAC Name:
2-phenyl-3H-quinazolin-4-one
InChI:
InChI=1S/C14H10N2O/c17-14-11-8-4-5-9-12(11)15-13(16-14)10-6-2-1-3-7-10/h1-9H,(H,15,16,17)
InChI Key:
VDULOAUXSMYUMG-UHFFFAOYSA-N
SMILES:
O=c1[nH]c(nc2c1cccc2)c1ccccc1
Properties
Complexity:
331
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
222.079g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
222.247g/mol
Monoisotopic Mass:
222.079g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
41.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.4
Literature
| Title | Journal |
|---|---|
| Computer-aided design, synthesis and validation of 2-phenylquinazolinone fragments as CDK9 inhibitors with anti-HIV-1 Tat-mediated transcription activity. | ChemMedChem 20131201 |
| Novel quinazolinone MJ-29 triggers endoplasmic reticulum stress and intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits leukemic mice. | PloS one 20120101 |
| Synthesis and cytotoxicity of 2-phenylquinazolin-4(3H)-one derivatives. | European journal of medicinal chemistry 20110901 |
| Synthesis, biological evaluation and molecular docking of quinazoline-4(1H)-one derivatives as anti-inflammatory and analgesic agents. | Acta poloniae pharmaceutica 20110101 |
| 2-Phenylquinazolin-4(3H)-one, a class of potent PDE5 inhibitors with high selectivity versus PDE6. | Bioorganic & medicinal chemistry letters 20090515 |
| Effect of active synthetic 2-substituted quinazolinones on anti-platelet aggregation and the inhibition of superoxide anion generation by neutrophils. | Archives of pharmacal research 20030701 |
Related Products
© 2019 Angene International Limited. All rights Reserved.