200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 10191-25-0
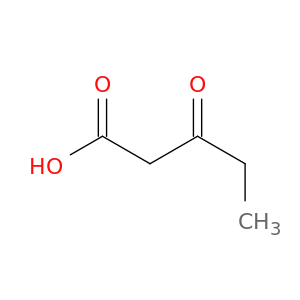
10191-25-0 | Pentanoic acid, 3-oxo-
CAS No: 10191-25-0 Catalog No: AG0005XF MDL No:MFCD11040434
Product Description
Catalog Number:
AG0005XF
Chemical Name:
Pentanoic acid, 3-oxo-
CAS Number:
10191-25-0
Molecular Formula:
C5H8O3
Molecular Weight:
116.1152
MDL Number:
MFCD11040434
IUPAC Name:
3-oxopentanoic acid
InChI:
InChI=1S/C5H8O3/c1-2-4(6)3-5(7)8/h2-3H2,1H3,(H,7,8)
InChI Key:
FHSUFDYFOHSYHI-UHFFFAOYSA-N
SMILES:
CCC(=O)CC(=O)O
UNII:
090PW368EP
Properties
Complexity:
106
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
116.047g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
116.116g/mol
Monoisotopic Mass:
116.047g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
54.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.1
Literature
| Title | Journal |
|---|---|
| Enzymatic functions of wild tomato methylketone synthases 1 and 2. | Plant physiology 20100901 |
| Interrelations between C4 ketogenesis, C5 ketogenesis, and anaplerosis in the perfused rat liver. | The Journal of biological chemistry 20091009 |
| Lowest transition state for the chirality-determining step in Ru((R)-BINAP)-catalyzed asymmetric hydrogenation of methyl-3-oxobutanoate. | Journal of the American Chemical Society 20081224 |
| (Z)-Methyl 3-(4-ethoxy-anilino)but-2-enoate. | Acta crystallographica. Section E, Structure reports online 20080601 |
| Volatile ketone formation in bacteria: release of 3-oxopentanoate by soil pseudomonads during growth on heptanoate. | Current microbiology 20010401 |
Related Products
© 2019 Angene International Limited. All rights Reserved.