200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1016-05-3
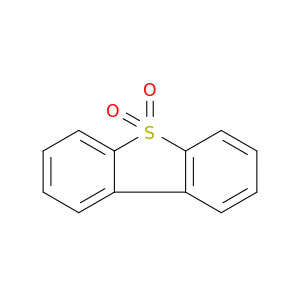
1016-05-3 | Dibenzothiophene, 5,5-dioxide
CAS No: 1016-05-3 Catalog No: AG0004YC MDL No:MFCD00004970
Product Description
Catalog Number:
AG0004YC
Chemical Name:
Dibenzothiophene, 5,5-dioxide
CAS Number:
1016-05-3
Molecular Formula:
C12H8O2S
Molecular Weight:
216.2557
MDL Number:
MFCD00004970
IUPAC Name:
dibenzothiophene 5,5-dioxide
InChI:
InChI=1S/C12H8O2S/c13-15(14)11-7-3-1-5-9(11)10-6-2-4-8-12(10)15/h1-8H
InChI Key:
IKJFYINYNJYDTA-UHFFFAOYSA-N
SMILES:
O=S1(=O)c2ccccc2c2c1cccc2
EC Number:
213-805-9
Properties
Complexity:
313
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
216.025g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
216.254g/mol
Monoisotopic Mass:
216.025g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
42.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.4
Literature
| Title | Journal |
|---|---|
| SAR of α7 nicotinic receptor agonists derived from tilorone: exploration of a novel nicotinic pharmacophore. | Bioorganic & medicinal chemistry letters 20120215 |
| The β phase formation limit in two poly(9,9-di-n-octylfluorene) based copolymers. | Macromolecular rapid communications 20110701 |
| π-conjugation and charge polarization in fluorene-dibenzothiophene-S,S-dioxide co-oligomers by Raman spectroscopy and quantum chemistry. | The Journal of chemical physics 20110128 |
| Tuning the intramolecular charge transfer emission from deep blue to green in ambipolar systems based on dibenzothiophene S,S-dioxide by manipulation of conjugation and strength of the electron donor units. | The Journal of organic chemistry 20101015 |
| The interplay of conformation and photophysical properties in deep-blue fluorescent oligomers. | Chemical communications (Cambridge, England) 20100714 |
| Dipolar stabilization of emissive singlet charge transfer excited states in polyfluorene copolymers. | The journal of physical chemistry. B 20080529 |
| Is there a homolytic substitution chemistry (SH2) of sulfones? | The Journal of organic chemistry 20050916 |
| Dibenzothiophene-S,S-dioxide-fluorene co-oligomers. Stable, highly-efficient blue emitters with improved electron affinity. | Chemical communications (Cambridge, England) 20050721 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Elucidation of the metabolic pathway of fluorene and cometabolic pathways of phenanthrene, fluoranthene, anthracene and dibenzothiophene by Sphingomonas sp. LB126. | Research in microbiology 20030401 |
| Elucidation of the metabolic pathway for dibenzothiophene desulphurization by Rhodococcus sp. strain IGTS8 (ATCC 53968). | Microbiology (Reading, England) 19970901 |
| Molecular mechanisms of biocatalytic desulfurization of fossil fuels. | Nature biotechnology 19961201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.