200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 10140-70-2
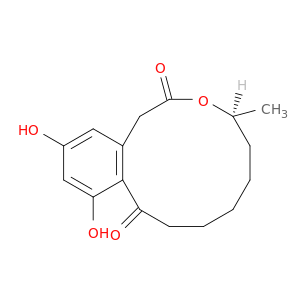
10140-70-2 | 2H-3-Benzoxacyclododecin-2,10(1H)-dione, 4,5,6,7,8,9-hexahydro-11,13-dihydroxy-4-methyl-, (4S)-
CAS No: 10140-70-2 Catalog No: AG0004ER MDL No:
Product Description
Catalog Number:
AG0004ER
Chemical Name:
2H-3-Benzoxacyclododecin-2,10(1H)-dione, 4,5,6,7,8,9-hexahydro-11,13-dihydroxy-4-methyl-, (4S)-
CAS Number:
10140-70-2
Molecular Formula:
C16H20O5
Molecular Weight:
292.3270
IUPAC Name:
(5S)-13,15-dihydroxy-5-methyl-4-oxabicyclo[10.4.0]hexadeca-1(12),13,15-triene-3,11-dione
InChI:
InChI=1S/C16H20O5/c1-10-5-3-2-4-6-13(18)16-11(8-15(20)21-10)7-12(17)9-14(16)19/h7,9-10,17,19H,2-6,8H2,1H3/t10-/m0/s1
InChI Key:
VDUIGYAPSXCJFC-JTQLQIEISA-N
SMILES:
C[C@H]1CCCCCC(=O)c2c(CC(=O)O1)cc(O)cc2O
UNII:
WT39K5T3BX
Properties
Complexity:
381
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
292.131g/mol
Formal Charge:
0
Heavy Atom Count:
21
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
292.331g/mol
Monoisotopic Mass:
292.131g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
83.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.1
Literature
| Title | Journal |
|---|---|
| [Biological activity of Penicillium sp. 10-51 exometabolites]. | Mikrobiolohichnyi zhurnal (Kiev, Ukraine : 1993) 20120101 |
| Aryne acyl-alkylation in the general and convergent synthesis of benzannulated macrolactone natural products: an enantioselective synthesis of (-)-curvularin. | Organic letters 20100402 |
| Transcriptional and post-transcriptional regulation of iNOS expression in human chondrocytes. | Biochemical pharmacology 20100301 |
| Isolation and difference in anti-Staphylococcus aureus bioactivity of curvularin derivates from fungus Eupenicillium sp. | Applied biochemistry and biotechnology 20091001 |
| Metabolite production by different Ulocladium species. | International journal of food microbiology 20080815 |
| Inhibitors of inducible NO synthase expression: total synthesis of (S)-curvularin and its ring homologues. | ChemMedChem 20080601 |
| First total syntheses and spectral data corrections of 11-alpha-methoxycurvularin and 11-beta-methoxycurvularin. | The Journal of organic chemistry 20071207 |
| A concise synthetic approach to beta,gamma-dehydrocurvularin: synthesis of (+/-)-di-O-methyl-beta,gamma-dehydrocurvularin. | Bioscience, biotechnology, and biochemistry 20070601 |
| Microbial transformation of curvularin. | Journal of natural products 20050801 |
| A new anthraquinone and cytotoxic curvularins of a Penicillium sp. from the rhizosphere of Fallugia paradoxa of the Sonoran desert. | The Journal of antibiotics 20040501 |
| Betagamma-dehydrocurvularin and related compounds as nematicides of Pratylenchus penetrans from the fungus Aspergillus sp. | Bioscience, biotechnology, and biochemistry 20030601 |
| Total synthesis of (s)-(+)-citreofuran by ring closing alkyne metathesis. | The Journal of organic chemistry 20030221 |
| Sporogen, S14-95, and S-curvularin, three inhibitors of human inducible nitric-oxide synthase expression isolated from fungi. | Molecular pharmacology 20030201 |
| Online analysis of xestodecalactones A-C, novel bioactive metabolites from the fungus Penicillium cf. montanense and their subsequent isolation from the sponge Xestospongia exigua. | Journal of natural products 20021101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.