200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1003-38-9
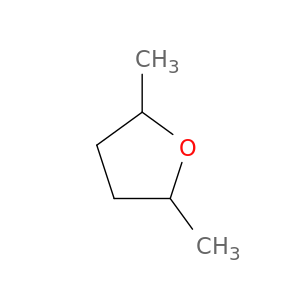
1003-38-9 | Furan, tetrahydro-2,5-dimethyl-
CAS No: 1003-38-9 Catalog No: AG0001IO MDL No:MFCD00005369
Product Description
Catalog Number:
AG0001IO
Chemical Name:
Furan, tetrahydro-2,5-dimethyl-
CAS Number:
1003-38-9
Molecular Formula:
C6H12O
Molecular Weight:
100.1589
MDL Number:
MFCD00005369
IUPAC Name:
2,5-dimethyloxolane
InChI:
InChI=1S/C6H12O/c1-5-3-4-6(2)7-5/h5-6H,3-4H2,1-2H3
InChI Key:
OXMIDRBAFOEOQT-UHFFFAOYSA-N
SMILES:
CC1CCC(O1)C
EC Number:
213-707-6
NSC Number:
12594
Properties
Complexity:
53.2
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
100.089g/mol
Formal Charge:
0
Heavy Atom Count:
7
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
100.161g/mol
Monoisotopic Mass:
100.089g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
9.2A^2
Undefined Atom Stereocenter Count:
2
Undefined Bond Stereocenter Count:
0
XLogP3:
1.2
Literature
| Title | Journal |
|---|---|
| Mechanistic study of a one-step catalytic conversion of fructose to 2,5-dimethyltetrahydrofuran. | Chemistry (Weinheim an der Bergstrasse, Germany) 20120924 |
| Kinetics and thermochemistry of 2,5-dimethyltetrahydrofuran and related oxolanes: next next-generation biofuels. | The journal of physical chemistry. A 20120510 |
| One-step catalytic transformation of carbohydrates and cellulosic biomass to 2,5-dimethyltetrahydrofuran for liquid fuels. | ChemSusChem 20100525 |
| Reactivity of niobium and tantalum pentahalides with cyclic ethers and the isolation and characterization of intermediates in the polymerization of tetrahydrofuran. | Inorganic chemistry 20080107 |
| The mechanism of epoxide carbonylation by [Lewis Acid]+[Co(CO)4]- catalysts. | Journal of the American Chemical Society 20060809 |
| Independent generation and study of 5,6-Dihydro-2'-deoxyuridin-6-yl, a member of the major family of reactive intermediates formed in DNA from the effects of gamma-radiolysis. | The Journal of organic chemistry 20030530 |
| Amine-chelated aryllithium reagents--structure and dynamics. | Journal of the American Chemical Society 20010822 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.