200,000+ products from a single source!
sales@angenechem.com
Home > General Informations and Structural Features of Boric Acid
General Informations and Structural Features of Boric Acid
General Informations and Structural Features of Boric Acid
In the past decade, the chemistry of boric acid has developed rapidly. The growing interest is mainly due to its mild and highly selective properties, as well as its environmentally beneficial properties and commercial availability. Boric acid is now commonly used as an effective acid catalyst in organic synthesis for various selective conversions of simple and complex molecules. The purpose of this review is to outline the use of boric acid, with a focus on recent synthetic applications. The literature is reported until the end of 2017.
Boric acid is a weak inorganic acid, also known as boric acid or orthoboric acid. It is a white crystalline solid with the following physical properties:
Molar mass: H3BO3 or B(OH) 3 molar mass: 61.83 g / mol
Density 1.435 g / cm3
Melting point: 170.9 oC (339.6 oF; 444.0 K)
Boiling point: 300 oC (572 oF; 573 K)
pKa: 9.24, 12.4, 13.3
Solubility: soluble in water and lower alcohol moderately soluble in pyridine; very slightly soluble in acetone
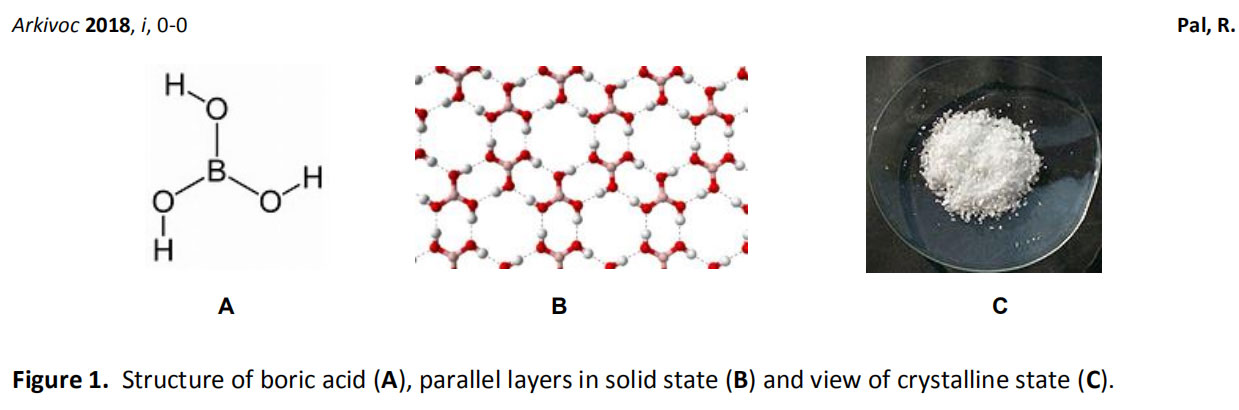
Figure 1A shows that the central boron atom is attached to three hydroxyl groups (-OH) which are capable of forming strong hydrogen bonds. Its solid crystal structure is held together by a parallel boric acid layer by hydrogen bonding (Fig. 1B). It is used as a source of weak monobasic acids. It has been used in various acid catalyzed reactions. It is easy to handle and safe to use because low concentrations of boric acid do not cause any toxicity. It is also used as a preservative, preservative, insecticide, pH buffer, swimming pool chemicals and precursors for many useful chemicals in acne treatment.
Esterification Reactions
Esterification is one of the oldest, most widely used and most important chemical transformations in organic synthesis, and is widely used in the chemical industry, pharmaceuticals, food, perfumes and cosmetics. These reactions can be used in the synthesis of natural products, in the protection or kinetic resolution of carboxylic acids, and in intramolecular reactions to prepare lactones. The esterification of carboxylic acids, hydroxy acids, and sugar acids has been studied using boric acid.
Carboxylic esterification
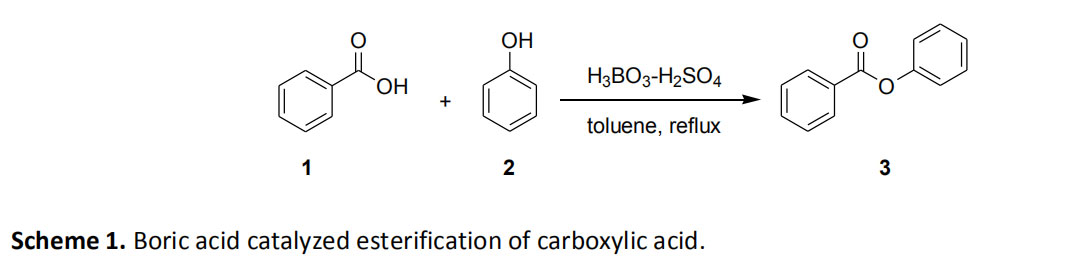
Direct esterification of phenols catalyzed by the combination of aliphatic and aromatic carboxylic acids with boric acid and sulfuric acid. Neither boric acid nor sulfuric acid can catalyze the reaction alone. Therefore, when azeotropic distillation is carried out by azeotropic distillation to remove water from azeotropic distillation of benzoic acid and a catalytic amount of boric acid and sulfuric acid in toluene, the yield of phenyl benzoate (3) is almost quantitative (Scheme 1). 1.
Esterification of α-hydroxy acid
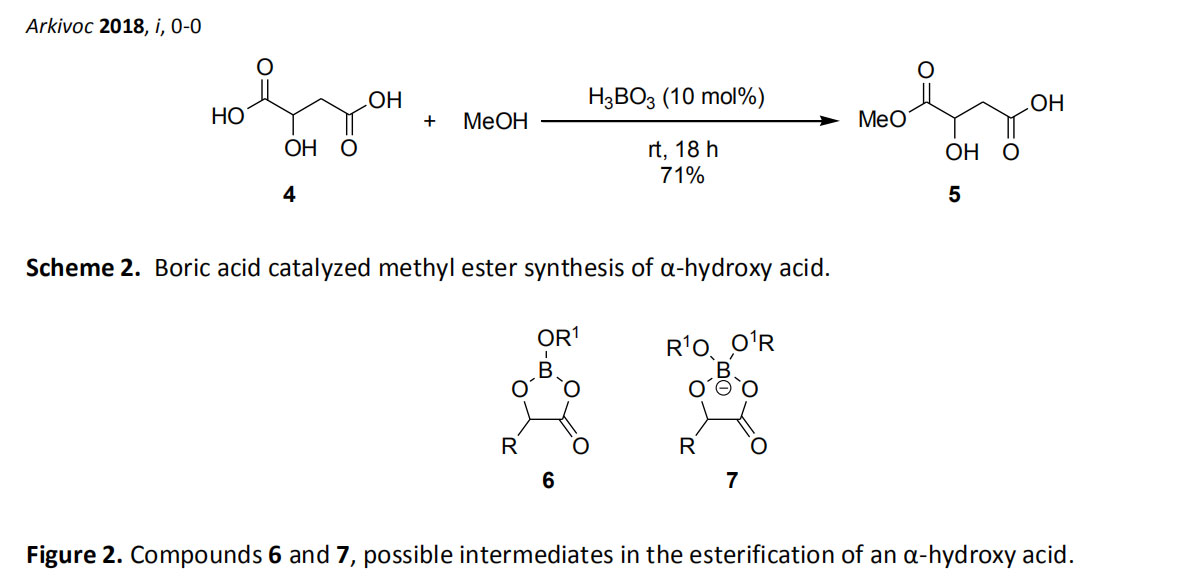
Houston and colleagues report the chemoselective esterification of alpha-hydroxy acids with boron. 2 when malic acid (4) (one relative to a carboxylic acid at the alpha position and relative to room temperature, the beta position relative to another) and methanol and boric acid are reacted overnight to obtain a high yield of single Ester 5 (Scheme 2). The -CO 2 H group relative to the β-hydroxy group remains unchanged. The effect of the α-OH group relative to the carboxylate is discussed. A five-membered cyclic neutral ester 6 and an anionic species 7 (Fig. 2) may be formed during the reaction to accelerate the esterification reaction.
Esterification of α-hydroxy acid
Houston and colleagues report the chemoselective esterification of alpha-hydroxy acids with boric acid. 2 when malic acid (4) (one relative to a carboxylic acid at the alpha position and relative to room temperature, the beta position relative to another) and methanol and boric acid are reacted overnight to obtain a high yield of single Ester 5 (Scheme 2). The -CO 2 H group relative to the β-hydroxy group remains unchanged. The effect of the α-OH group relative to the carboxylate is discussed. A five-membered cyclic neutral ester 6 and an anionic species 7 (Fig. 2) may be formed during the reaction to accelerate the esterification reaction.
Esterification of sugar acids
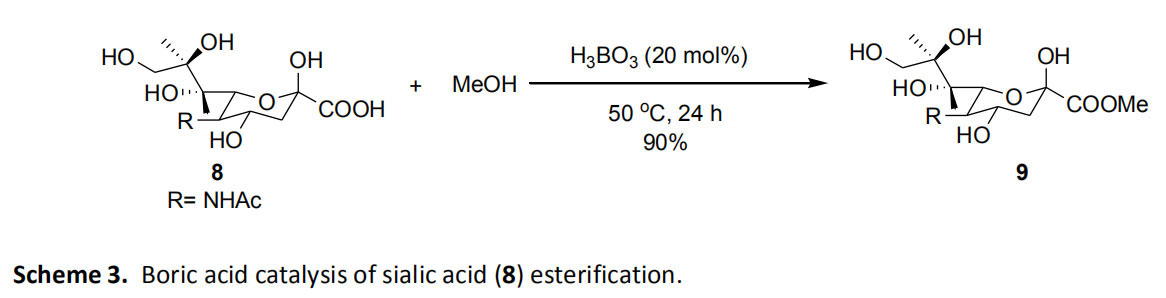
The sugar has been esterified with methanol in the presence of boric acid. Therefore, when the sialic acid (8) (a sugar acid) is treated with anhydrous methanol under a nitrogen atmosphere at a temperature of 50 ° C in the presence of a catalytic amount of boric acid, the yield of the produced methyl ester 9 is 90% (scheme) 3). 3 The reaction proceeds with high efficiency in certain sugar acid molecules, but is highly dependent on the substrate. Glucuronic acid containing a β-hydroxycarboxylate motif and bacterial monosaccharide 3-deoxy-D-mannan-oct-2-ulosonic acid (KDO) containing an α-hydroxycarboxylate motif in methanol It cannot be esterified by boric acid catalysis.
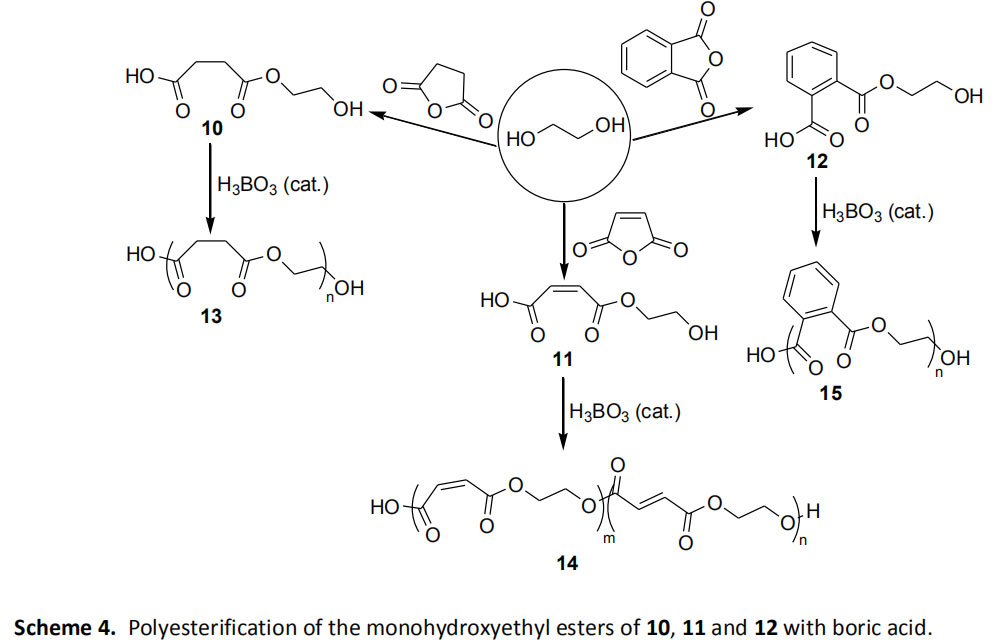
Esterification of monocarboxylic acid monohydroxyethyl ester
Alemdar et al. [4] found that a boric acid-pyridine mixture can be used as a mild catalyst for the polyester reaction of succinic acid succinic acid 10, maleic acid 11 and monohydroxyethyl phthalate 12 . The catalyst system was shown to provide a colorless polyester 13, 14, 15 for low molecular weight (M: 1650-1950) within 4 hours at 130 °C (Scheme 4).
© 2019 Angene International Limited. All rights Reserved.


