200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 95044-71-6
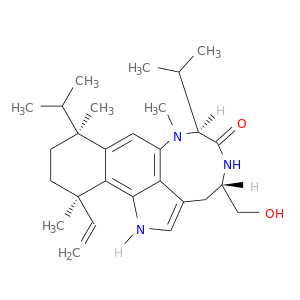
95044-71-6 | (4s,7s,10s,13r)-13-ethenyl-4-(hydroxymethyl)-8,10,13-trimethyl-7,10-di(propan-2-yl)-1,3,4,5,7,8,10,11,12,13-decahydro-6h-benzo[g][1,4]diazonino[7,6,5-cd]indol-6-one
CAS No: 95044-71-6 Catalog No: AG005TJ1 MDL No:
Product Description
Catalog Number:
AG005TJ1
Chemical Name:
(4s,7s,10s,13r)-13-ethenyl-4-(hydroxymethyl)-8,10,13-trimethyl-7,10-di(propan-2-yl)-1,3,4,5,7,8,10,11,12,13-decahydro-6h-benzo[g][1,4]diazonino[7,6,5-cd]indol-6-one
CAS Number:
95044-71-6
Molecular Formula:
C28H41N3O2
Molecular Weight:
451.6440
IUPAC Name:
(6S,9S,14S,17R)-17-ethenyl-6-(hydroxymethyl)-10,14,17-trimethyl-9,14-di(propan-2-yl)-2,7,10-triazatetracyclo[9.7.1.04,19.013,18]nonadeca-1(18),3,11(19),12-tetraen-8-one
InChI:
InChI=1S/C28H41N3O2/c1-9-27(6)10-11-28(7,17(4)5)20-13-21-22-18(14-29-24(22)23(20)27)12-19(15-32)30-26(33)25(16(2)3)31(21)8/h9,13-14,16-17,19,25,29,32H,1,10-12,15H2,2-8H3,(H,30,33)/t19-,25-,27-,28-/m0/s1
InChI Key:
PEYTUVXFLCCGCC-VCJXGGRGSA-N
SMILES:
OC[C@H]1NC(=O)[C@H](C(C)C)N(c2c3c(C1)c[nH]c3c1c(c2)[C@@](C)(CC[C@]1(C)C=C)C(C)C)C
UNII:
M3CR2EV9W8
Properties
Complexity:
753
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
451.32g/mol
Formal Charge:
0
Heavy Atom Count:
33
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
451.655g/mol
Monoisotopic Mass:
451.32g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
68.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
6.5
Literature
| Title | Journal |
|---|---|
| Generation of 'Unnatural Natural Product' library and identification of a small molecule inhibitor of XIAP. | Bioorganic & medicinal chemistry 20110715 |
| Activity of mangosteen xanthones and teleocidin a-2 in death receptor expression enhancement and tumor necrosis factor related apoptosis-inducing ligand assays. | Journal of natural products 20100326 |
| A new teleocidin analog from Streptomyces sp. MM216-87F4 induces substance P release from rat dorsal root ganglion neurons. | The Journal of antibiotics 20060101 |
| C-C bond formation via C-H bond activation: synthesis of the core of teleocidin B4. | Journal of the American Chemical Society 20021009 |
| Synthesis of 7,8-disubstituted benzolactam-V8 and its binding to protein kinase C. | Bioorganic & medicinal chemistry letters 20010122 |
| Teleocidins and benzolactams inhibit cell killing by human immunodeficiency virus type 1 (HIV-1). | Biological & pharmaceutical bulletin 19940801 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







