200,000+ products from a single source!
sales@angenechem.com
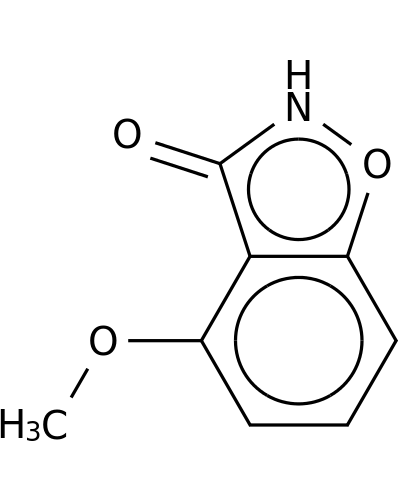
Home > Indoles and Oxindole > 94944-80-6
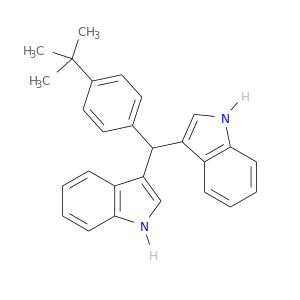
94944-80-6 | 3,3'-[[4-(TERT-BUTYL)PHENYL]METHYLENE]BIS(1H-INDOLE)
CAS No: 94944-80-6 Catalog No: AG005U22 MDL No:
Product Description
Catalog Number:
AG005U22
Chemical Name:
3,3'-[[4-(TERT-BUTYL)PHENYL]METHYLENE]BIS(1H-INDOLE)
CAS Number:
94944-80-6
Molecular Formula:
C27H26N2
Molecular Weight:
378.5087
IUPAC Name:
3-[(4-tert-butylphenyl)-(1H-indol-3-yl)methyl]-1H-indole
InChI:
InChI=1S/C27H26N2/c1-27(2,3)19-14-12-18(13-15-19)26(22-16-28-24-10-6-4-8-20(22)24)23-17-29-25-11-7-5-9-21(23)25/h4-17,26,28-29H,1-3H3
InChI Key:
NCPVEZRBHNDCIV-UHFFFAOYSA-N
SMILES:
CC(c1ccc(cc1)C(c1c[nH]c2c1cccc2)c1c[nH]c2c1cccc2)(C)C
EC Number:
305-658-5
Properties
Complexity:
511
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
378.21g/mol
Formal Charge:
0
Heavy Atom Count:
29
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
378.519g/mol
Monoisotopic Mass:
378.21g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
31.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
7.2
Literature
| Title | Journal |
|---|---|
| 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes induce autophagic cell death in estrogen receptor negative breast cancer. | BMC cancer 20100101 |
| Suppression of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nitric-oxide synthase 2 expression in astrocytes by a novel diindolylmethane analog protects striatal neurons against apoptosis. | Molecular pharmacology 20090101 |
| Cancer chemotherapy with indole-3-carbinol, bis(3'-indolyl)methane and synthetic analogs. | Cancer letters 20081008 |
| 1,1-bis(3'-indolyl)-1-(p-substituted phenyl)methanes decrease mitochondrial membrane potential and induce apoptosis in endometrial and other cancer cell lines. | Molecular carcinogenesis 20080701 |
| 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit proliferation of estrogen receptor-negative breast cancer cells by activation of multiple pathways. | Breast cancer research and treatment 20080501 |
| 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl)methanes induce apoptosis and inhibit renal cell carcinoma growth. | Clinical cancer research : an official journal of the American Association for Cancer Research 20071115 |
| 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit ovarian cancer cell growth through peroxisome proliferator-activated receptor-dependent and independent pathways. | Molecular cancer therapeutics 20060901 |
| 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through PPARgamma-dependent and PPARgamma-independent pathways. | Molecular cancer therapeutics 20060501 |
| Inhibition of bladder tumor growth by 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor gamma agonists. | Cancer research 20060101 |
| 1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and nonsteroidal anti-inflammatory drug-activated gene-1. | Molecular pharmacology 20051201 |
| Inhibition of tumor-necrosis-factor-alpha induced endothelial cell activation by a new class of PPAR-gamma agonists. An in vitro study showing receptor-independent effects. | Journal of vascular research 20050101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.