200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 948-71-0
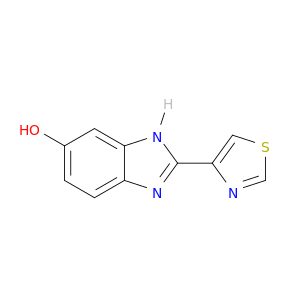
948-71-0 | 2-(4-thiazolyl)-5-benzimidazolol
CAS No: 948-71-0 Catalog No: AG0067B4 MDL No:MFCD00152219
Product Description
Catalog Number:
AG0067B4
Chemical Name:
2-(4-thiazolyl)-5-benzimidazolol
CAS Number:
948-71-0
Molecular Formula:
C10H7N3OS
Molecular Weight:
217.2471
MDL Number:
MFCD00152219
IUPAC Name:
2-(1,3-thiazol-4-yl)-3H-benzimidazol-5-ol
InChI:
InChI=1S/C10H7N3OS/c14-6-1-2-7-8(3-6)13-10(12-7)9-4-15-5-11-9/h1-5,14H,(H,12,13)
InChI Key:
VNENJHUOPQAPAT-UHFFFAOYSA-N
SMILES:
Oc1ccc2c(c1)[nH]c(n2)c1cscn1
Properties
Complexity:
241
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
217.031g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
217.246g/mol
Monoisotopic Mass:
217.031g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
90A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.1
Literature
| Title | Journal |
|---|---|
| Adaptive and specialised transcriptional responses to xenobiotic stress in Caenorhabditis elegans are regulated by nuclear hormone receptors. | PloS one 20130101 |
| Myeloperoxidase-mediated bioactivation of 5-hydroxythiabendazole: a possible mechanism of thiabendazole toxicity. | Toxicology in vitro : an international journal published in association with BIBRA 20110801 |
| [Distribution and elimination of thiabendazole and its metabolite residue in laying hens]. | Wei sheng yan jiu = Journal of hygiene research 20110501 |
| In vitro and in silico identification and characterization of thiabendazole as a mechanism-based inhibitor of CYP1A2 and simulation of possible pharmacokinetic drug-drug interactions. | Drug metabolism and disposition: the biological fate of chemicals 20090601 |
| In vitro metabolic activation of thiabendazole via 5-hydroxythiabendazole: identification of a glutathione conjugate of 5-hydroxythiabendazole. | Drug metabolism and disposition: the biological fate of chemicals 20060401 |
| Evidence for cytochrome P4501A2-mediated protein covalent binding of thiabendazole and for its passive intestinal transport: use of human and rabbit derived cells. | Chemico-biological interactions 20000703 |
Related Products
© 2019 Angene International Limited. All rights Reserved.