200,000+ products from a single source!
sales@angenechem.com
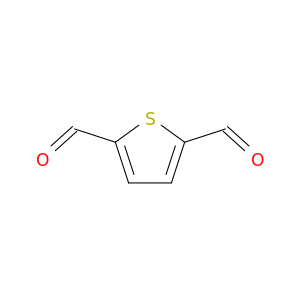
932-95-6 | Thiophene-2,5-dicarbaldehyde
CAS No: 932-95-6 Catalog No: AG003G1E MDL No:MFCD00216592
Product Description
Catalog Number:
AG003G1E
Chemical Name:
Thiophene-2,5-dicarbaldehyde
CAS Number:
932-95-6
Molecular Formula:
C6H4O2S
Molecular Weight:
140.1598
MDL Number:
MFCD00216592
IUPAC Name:
thiophene-2,5-dicarbaldehyde
InChI:
InChI=1S/C6H4O2S/c7-3-5-1-2-6(4-8)9-5/h1-4H
InChI Key:
OTMRXENQDSQACG-UHFFFAOYSA-N
SMILES:
O=Cc1ccc(s1)C=O
Properties
Complexity:
110
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
139.993g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
140.156g/mol
Monoisotopic Mass:
139.993g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
62.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.2
Literature
| Title | Journal |
|---|---|
| (E)-1-Phenyl-2-({5-[(1E)-(2-phenyl-hydrazin-1-yl-idene)meth-yl]-2-thien-yl}methyl-idene)hydrazine. | Acta crystallographica. Section E, Structure reports online 20100301 |
| 2-[5-(Benzo[d]thia-zol-2-yl)thio-phen-2-yl]benzo[d]thia-zole. | Acta crystallographica. Section E, Structure reports online 20100301 |
| 5-{[(E)-2-(4-Iodo-phen-yl)hydrazinyl-idene]meth-yl}thio-phene-2-carbaldehyde. | Acta crystallographica. Section E, Structure reports online 20100201 |
| (E)-1-(3-Nitro-phen-yl)-2-({5-[(1E)-2-(3-nitro-phen-yl)hydrazin-1-ylidenemeth-yl]-2-thien-yl}methyl-idene)hydrazine. | Acta crystallographica. Section E, Structure reports online 20100201 |
| Synthesis of some thiophene, imidazole and pyridine derivatives exhibiting good anti-inflammatory and analgesic activities. | Medicinal chemistry (Shariqah (United Arab Emirates)) 20080301 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Oxidation of heterocyclic and aromatic aldehydes to the corresponding carboxylic acids by Acetobacter and Serratia strains. | Biotechnology letters 20041101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.