200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 90154-87-3
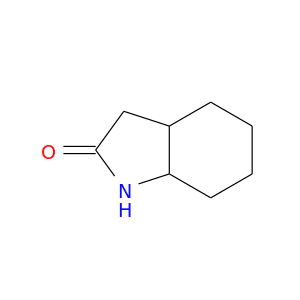
90154-87-3 | octahydro-1H-indol-2-one
CAS No: 90154-87-3 Catalog No: AG01BFXB MDL No:MFCD18449901
Product Description
Catalog Number:
AG01BFXB
Chemical Name:
octahydro-1H-indol-2-one
CAS Number:
90154-87-3
Molecular Formula:
C8H13NO
Molecular Weight:
139.1949
MDL Number:
MFCD18449901
IUPAC Name:
1,3,3a,4,5,6,7,7a-octahydroindol-2-one
InChI:
InChI=1S/C8H13NO/c10-8-5-6-3-1-2-4-7(6)9-8/h6-7H,1-5H2,(H,9,10)
InChI Key:
GFOOVKOSROWXAZ-UHFFFAOYSA-N
SMILES:
O=C1CC2C(N1)CCCC2
Properties
Complexity:
155
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
139.1g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
139.198g/mol
Monoisotopic Mass:
139.1g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
29.1A^2
Undefined Atom Stereocenter Count:
2
Undefined Bond Stereocenter Count:
0
XLogP3:
1
Literature
| Title | Journal |
|---|---|
| An IMDAF cycloaddition approach toward the synthesis of the lycopodium alkaloid (±)-fawcettidine. | The Journal of organic chemistry 20110415 |
| Construction of perhydro indol-2-ones by a methoxide catalyzed deacetylation-Michael-aldol cascade. | Chemical communications (Cambridge, England) 20100314 |
| Synthesis of some members of the hydroxylated phenanthridone subclass of the Amaryllidaceae alkaloid family. | The Journal of organic chemistry 20070330 |
| Chemistry of the Hexahydropyrrolo[2,3-b]indoles: configuration, conformation, reactivity, and applications in synthesis. | Accounts of chemical research 20070201 |
| An aza-Wittig/pi-furan cyclization approach toward the homoerythrina alkaloid (+/-)-selaginoidine. | Organic letters 20050331 |
| A study of vinyl radical cyclization using N-alkenyl-7-bromo-substituted hexahydroindolinones. | The Journal of organic chemistry 20050121 |
| Electrophilic-induced cyclization reaction of hexahydroindolinone derivatives and its application toward the synthesis of (+/-)-erysotramidine. | The Journal of organic chemistry 20041126 |
| Rh(I)-catalyzed ring opening of an IMDAF-derived oxabicyclo cycloadduct as the key step in the synthesis of (+/-)-epi-zephyranthine. | Organic letters 20040624 |
| Efficient synthesis of (+/-)-erysotramidine using an NBS-promoted cyclization reaction of a hexahydroindolinone derivative. | Organic letters 20031225 |
| Application of furanyl carbamate cycloadditions toward the synthesis of hexahydroindolinone alkaloids. | The Journal of organic chemistry 20010504 |
| Formal total synthesis of (+/-)-gamma-lycorane and (+/-)-1-deoxylycorine using the [4+2]-cycloaddition/rearrangement cascade of furanyl carbamates. | The Journal of organic chemistry 20010309 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.