200,000+ products from a single source!
sales@angenechem.com
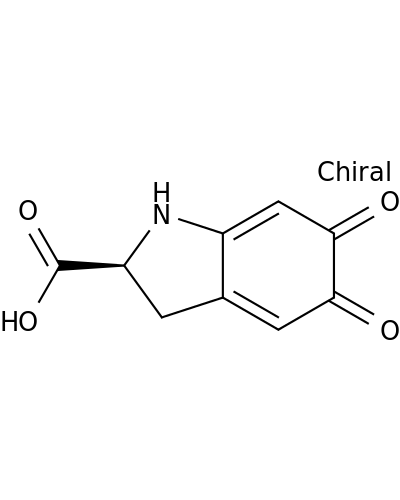
89762-39-0 | 1H-Indole-2-carboxylicacid, 2,3,5,6-tetrahydro-5,6-dioxo-, (2S)-
CAS No: 89762-39-0 Catalog No: AG003ZRL MDL No:
Product Description
Catalog Number:
AG003ZRL
Chemical Name:
1H-Indole-2-carboxylicacid, 2,3,5,6-tetrahydro-5,6-dioxo-, (2S)-
CAS Number:
89762-39-0
Molecular Formula:
C9H7NO4
Molecular Weight:
193.1562
IUPAC Name:
5,6-dioxo-2,3-dihydro-1H-indole-2-carboxylic acid
InChI:
InChI=1S/C9H7NO4/c11-7-2-4-1-6(9(13)14)10-5(4)3-8(7)12/h2-3,6,10H,1H2,(H,13,14)
InChI Key:
VJNCICVKUHKIIV-UHFFFAOYSA-N
SMILES:
OC(=O)[C@H]1NC2=CC(=O)C(=O)C=C2C1
Properties
Complexity:
405
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
193.038g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
193.158g/mol
Monoisotopic Mass:
193.038g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
83.5A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.7
Literature
| Title | Journal |
|---|---|
| Quercetin-3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside suppresses melanin synthesis by augmenting p38 MAPK and CREB signaling pathways and subsequent cAMP down-regulation in murine melanoma cells. | Saudi journal of biological sciences 20151101 |
| Extracellular tyrosinase from the fungus Trichoderma reesei shows product inhibition and different inhibition mechanism from the intracellular tyrosinase from Agaricus bisporus. | Biochimica et biophysica acta 20120401 |
| Tyrosinase inhibition by water and ethanol extracts of a far eastern sea cucumber, Stichopus japonicus. | Journal of the science of food and agriculture 20110701 |
| Biocatalytic formation of synthetic melanin: the role of vanadium haloperoxidases, L-DOPA and iodide. | Journal of inorganic biochemistry 20110601 |
| Cytotoxicity of dopaminochrome in the mesencephalic cell line, MN9D, is dependent upon oxidative stress. | Neurotoxicology 20091101 |
| Phenoloxidase activity in three commercial bivalve species. Changes due to natural infestation with Perkinsus atlanticus. | Fish & shellfish immunology 20060101 |
| A novel mechanism in control of human pigmentation by {beta}-melanocyte-stimulating hormone and 7-tetrahydrobiopterin. | The Journal of endocrinology 20051101 |
| Involvement of calpain in melanogenesis of mouse B16 melanoma cells. | Molecular and cellular biochemistry 20050701 |
| [UV-vis spectroscopic study of the effect of Cu(II) ions on dopachrome]. | Guang pu xue yu guang pu fen xi = Guang pu 20040701 |
| Aluminum ions accelerated the oxidative stress of copper-mediated melanin formation. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20031101 |
| Rate constants for the first two chemical steps of eumelanogenesis. | Pigment cell research 20031001 |
| Effect of aluminum (III) on the conversion of dopachrome in the melanin synthesis pathway. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20030601 |
| UVB-induced inflammation gives increased d-dopachrome tautomerase activity in blister fluid which correlates with macrophage migration inhibitory factor. | Experimental dermatology 20030601 |
| Chemical and biological aspects of melanin. | The Alkaloids. Chemistry and biology 20030101 |
| Identification of Drosophila melanogaster yellow-f and yellow-f2 proteins as dopachrome-conversion enzymes. | The Biochemical journal 20021115 |
| Behavioral effects of aminochrome and dopachrome injected in the rat substantia nigra. | Pharmacology, biochemistry, and behavior 20021101 |
| Tyrosinase kinetics: a semi-quantitative model of the mechanism of oxidation of monohydric and dihydric phenolic substrates--reply. | Journal of theoretical biology 20020121 |
| Functional expression and characterization of Aedes aegypti dopachrome conversion enzyme. | Biochemical and biophysical research communications 20020111 |
| Cloning and characterization of a dopachrome conversion enzyme from the yellow fever mosquito, Aedes aegypti. | Insect biochemistry and molecular biology 20011001 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







