200,000+ products from a single source!
sales@angenechem.com
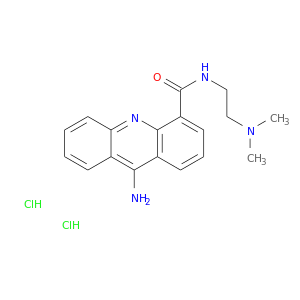
89459-43-8 | 4-Acridinecarboxamide,9-amino-N-[2-(dimethylamino)ethyl]-
CAS No: 89459-43-8 Catalog No: AG0042JT MDL No:
Product Description
Catalog Number:
AG0042JT
Chemical Name:
4-Acridinecarboxamide,9-amino-N-[2-(dimethylamino)ethyl]-
CAS Number:
89459-43-8
Molecular Formula:
C18H22Cl2N4O
Molecular Weight:
381.2995
IUPAC Name:
9-amino-N-[2-(dimethylamino)ethyl]acridine-4-carboxamide
InChI:
InChI=1S/C18H20N4O/c1-22(2)11-10-20-18(23)14-8-5-7-13-16(19)12-6-3-4-9-15(12)21-17(13)14/h3-9H,10-11H2,1-2H3,(H2,19,21)(H,20,23)
InChI Key:
YLGMVQJPGUHTRO-UHFFFAOYSA-N
SMILES:
CN(CCNC(=O)c1cccc2c1nc1ccccc1c2N)C.Cl.Cl
Properties
Complexity:
414
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
308.164g/mol
Formal Charge:
0
Heavy Atom Count:
23
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
308.385g/mol
Monoisotopic Mass:
308.164g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
71.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.2
Literature
| Title | Journal |
|---|---|
| Intracellular trafficking as a determinant of AS-DACA cytotoxicity in rhabdomyosarcoma cells. | BMC cell biology 20110101 |
| Attenuation of cytotoxic natural product DNA intercalating agents by caffeine. | Scientia pharmaceutica 20110101 |
| DNA threading bis(9-aminoacridine-4-carboxamides): effects of piperidine sidechains on DNA binding, cytotoxicity and cell cycle arrest. | Bioorganic & medicinal chemistry 20080415 |
| Influence of substituent modifications on DNA binding energetics of acridine-based anticancer agents. | Biochemistry 20031125 |
| Kinetic studies of the binding of acridinecarboxamide topoisomerase poisons to DNA: implications for mode of binding of ligands with uncharged chromophores. | Journal of medicinal chemistry 20020214 |
| Anticancer, antiradical and antioxidative actions of novel Antoksyd S and its major components, baicalin and baicalein. | Anticancer research 20020101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.