200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 89371-44-8
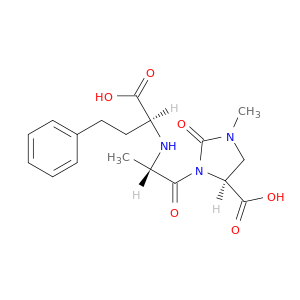
89371-44-8 | Imidaprilat
CAS No: 89371-44-8 Catalog No: AG003XKL MDL No:
Product Description
Catalog Number:
AG003XKL
Chemical Name:
Imidaprilat
CAS Number:
89371-44-8
Molecular Formula:
C18H23N3O6
Molecular Weight:
377.3917
IUPAC Name:
(4S)-3-[(2S)-2-[[(1S)-1-carboxy-3-phenylpropyl]amino]propanoyl]-1-methyl-2-oxoimidazolidine-4-carboxylic acid
InChI:
InChI=1S/C18H23N3O6/c1-11(15(22)21-14(17(25)26)10-20(2)18(21)27)19-13(16(23)24)9-8-12-6-4-3-5-7-12/h3-7,11,13-14,19H,8-10H2,1-2H3,(H,23,24)(H,25,26)/t11-,13-,14-/m0/s1
InChI Key:
VFAVNRVDTAPBNR-UBHSHLNASA-N
SMILES:
C[C@@H](C(=O)N1C(=O)N(C[C@H]1C(=O)O)C)N[C@H](C(=O)O)CCc1ccccc1
UNII:
WUU07Y30IA
Properties
Complexity:
590
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
377.159g/mol
Formal Charge:
0
Heavy Atom Count:
27
Hydrogen Bond Acceptor Count:
7
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
377.397g/mol
Monoisotopic Mass:
377.159g/mol
Rotatable Bond Count:
8
Topological Polar Surface Area:
127A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-1.4
Literature
| Title | Journal |
|---|---|
| Aliskiren inhibits intracellular angiotensin II levels without affecting (pro)renin receptor signals in human podocytes. | American journal of hypertension 20100501 |
| Different inhibitory effects in rat and human carboxylesterases. | Drug metabolism and disposition: the biological fate of chemicals 20090501 |
| Angiotensin-converting enzyme inhibitor attenuates monocyte adhesion to vascular endothelium through modulation of intracellular zinc. | The Journal of pharmacology and experimental therapeutics 20071201 |
| Imidapril: a review of its use in essential hypertension, Type 1 diabetic nephropathy and chronic heart failure. | Drugs 20070101 |
| Imidaprilat suppresses nonylphenol and 1-methyl-4-phenylpyridinium ion (MPP+)-induced hydroxyl radical generation in rat striatum. | Neuroscience research 20060301 |
| Imidaprilat, an angiotensin-converting enzyme inhibitor exerts neuroprotective effect via decreasing dopamine efflux and hydroxyl radical generation induced by bisphenol A and MPP+ in rat striatum. | Brain research 20060203 |
| Protective effect of imidaprilat, an angiotensin-converting enzyme inhibitor on *OH generation in rat myocardium. | Biochimica et biophysica acta 19991018 |
| No relation of the suppressive effect on the sympathetic nervous system to the acute hypotension caused by imidapril and enalapril. | Japanese journal of pharmacology 19931101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.