200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 89-56-5
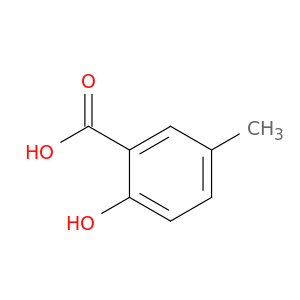
89-56-5 | 5-Methylsalicylic acid
CAS No: 89-56-5 Catalog No: AG003ZC6 MDL No:MFCD00002461
Product Description
Catalog Number:
AG003ZC6
Chemical Name:
5-Methylsalicylic acid
CAS Number:
89-56-5
Molecular Formula:
C8H8O3
Molecular Weight:
152.1473
MDL Number:
MFCD00002461
IUPAC Name:
2-hydroxy-5-methylbenzoic acid
InChI:
InChI=1S/C8H8O3/c1-5-2-3-7(9)6(4-5)8(10)11/h2-4,9H,1H3,(H,10,11)
InChI Key:
DLGBEGBHXSAQOC-UHFFFAOYSA-N
SMILES:
Cc1ccc(c(c1)C(=O)O)O
EC Number:
201-918-6
UNII:
6GAI2MTV5V
NSC Number:
38518
Properties
Complexity:
155
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
152.047g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
152.149g/mol
Monoisotopic Mass:
152.047g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
57.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.8
Literature
| Title | Journal |
|---|---|
| Quantitative correlation of counterion (X) affinity to ionic micelles and X- and temperature-induced micellar growth (spherical-wormlike micelles-vesicles) for X = 5-methyl- and 5-methoxysalicylate ions. | The journal of physical chemistry. B 20120223 |
| Fluorescence-based high-throughput screening assay for drug interactions with UGT1A6. | Assay and drug development technologies 20111001 |
| Synthesis of gadolinium nanoscale metal-organic framework with hydrotropes: manipulation of particle size and magnetic resonance imaging capability. | ACS applied materials & interfaces 20110501 |
| Identifying chelators for metalloprotein inhibitors using a fragment-based approach. | Journal of medicinal chemistry 20110127 |
| Self-assembly of surfactant vesicles that transform into viscoelastic wormlike micelles upon heating. | Journal of the American Chemical Society 20060524 |
| Catabolic role of a three-component salicylate oxygenase from Sphingomonas yanoikuyae B1 in polycyclic aromatic hydrocarbon degradation. | Biochemical and biophysical research communications 20050218 |
| Biochemical and molecular characterization of a ring fission dioxygenase with the ability to oxidize (substituted) salicylate(s) from Pseudaminobacter salicylatoxidans. | The Journal of biological chemistry 20040903 |
Related Products
© 2019 Angene International Limited. All rights Reserved.