200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 88426-33-9
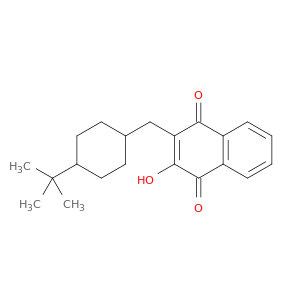
88426-33-9 | Buparvaquone
CAS No: 88426-33-9 Catalog No: AG004ESQ MDL No:MFCD01712789
Product Description
Catalog Number:
AG004ESQ
Chemical Name:
Buparvaquone
CAS Number:
88426-33-9
Molecular Formula:
C21H26O3
Molecular Weight:
326.4293
MDL Number:
MFCD01712789
IUPAC Name:
3-[(4-tert-butylcyclohexyl)methyl]-4-hydroxynaphthalene-1,2-dione
InChI:
InChI=1S/C21H26O3/c1-21(2,3)14-10-8-13(9-11-14)12-17-18(22)15-6-4-5-7-16(15)19(23)20(17)24/h4-7,13-14,22H,8-12H2,1-3H3
InChI Key:
NEGDTWQGGLJCTL-UHFFFAOYSA-N
SMILES:
OC1=C(CC2CCC(CC2)C(C)(C)C)C(=O)c2c(C1=O)cccc2
UNII:
0354RT7LG4
Properties
Complexity:
544
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
326.188g/mol
Formal Charge:
0
Heavy Atom Count:
24
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
326.436g/mol
Monoisotopic Mass:
326.188g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
54.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
5.3
Literature
| Title | Journal |
|---|---|
| Drug delivery systems for the topical treatment of cutaneous leishmaniasis. | Expert opinion on drug delivery 20120901 |
| Point mutations in the Theileria annulata cytochrome b gene is associated with buparvaquone treatment failure. | Veterinary parasitology 20120706 |
| Effectiveness of liposomal buparvaquone in an experimental hamster model of Leishmania (L.) infantum chagasi. | Experimental parasitology 20120301 |
| Evaluation and structure-activity relationship study of acute toxicity of naphthoquinones to Photobacterium phosphoreum, Photobacterium T3B. | Bulletin of environmental contamination and toxicology 20100801 |
| In vitro and in vivo evaluation of self-microemulsifying drug delivery system of buparvaquone. | Drug development and industrial pharmacy 20100601 |
| Cytoplasmic sequestration of p53 promotes survival in leukocytes transformed by Theileria. | Oncogene 20100527 |
| In vivo evidence for the resistance of Theileria annulata to buparvaquone. | Veterinary parasitology 20100511 |
| The protozoan parasite Theileria annulata alters the differentiation state of the infected macrophage and suppresses musculoaponeurotic fibrosarcoma oncogene (MAF) transcription factors. | International journal for parasitology 20090801 |
| Optimization and validation of RP-HPLC-UV method with solid-phase extraction for determination of buparvaquone in human and rabbit plasma: application to pharmacokinetic study. | Biomedical chromatography : BMC 20080501 |
| [Therapeutic efficacy of buparvaquone (buparvon) in cattle with theileriosis]. | Turkiye parazitolojii dergisi 20080101 |
| In vivo studies on the antileishmanial activity of buparvaquone and its prodrugs. | The Journal of antimicrobial chemotherapy 20071001 |
| Clinical, haematological and therapeutic studies on tropical theileriosis in water buffaloes (Bubalus bubalis) in Egypt. | Veterinary parasitology 20070531 |
| Development and validation of RP-HPLC-UV method for simultaneous determination of buparvaquone, atenolol, propranolol, quinidine and verapamil: a tool for the standardization of rat in situ intestinal permeability studies. | Journal of pharmaceutical and biomedical analysis 20070312 |
| Topical buparvaquone formulations for the treatment of cutaneous leishmaniasis. | The Journal of pharmacy and pharmacology 20070101 |
| Clinical efficacy and plasma concentrations of two formulations of buparvaquone in cattle infected with East Coast fever (Theileria parva infection). | Research in veterinary science 20060801 |
| Evaluation of buparvaquone (BUTA-Kel KELA, Belgium) as a treatment of East Coast fever in cattle, in the peri-urban of Dar Es Salaam city, Tanzania. | Veterinary parasitology 20060630 |
| Characterization of nebulized buparvaquone nanosuspensions--effect of nebulization technology. | Journal of drug targeting 20050101 |
| Design, synthesis and in vitro evaluation of novel water-soluble prodrugs of buparvaquone. | European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 20041001 |
| Synthesis and antileishmanial activity of novel buparvaquone oxime derivatives. | Bioorganic & medicinal chemistry 20040701 |
| The effect of dexamethasone and promethazine in combination with buparvaquone in the management of East Coast fever. | The Onderstepoort journal of veterinary research 20040601 |
| Cerebral theileriosis in a Holstein calf. | The Veterinary record 20040424 |
| Synthesis, in vitro evaluation, and antileishmanial activity of water-soluble prodrugs of buparvaquone. | Journal of medicinal chemistry 20040101 |
| In-vivo therapeutic efficacy trial with artemisinin derivative, buparvaquone and imidocarb dipropionate against Babesia equi infection in donkeys. | The Journal of veterinary medical science 20031101 |
| Theileria parva-transformed T cells show enhanced resistance to Fas/Fas ligand-induced apoptosis. | Journal of immunology (Baltimore, Md. : 1950) 20030801 |
| Apoptosis of Theileria-infected lymphocytes induced upon parasite death involves activation of caspases 9 and 3. | Biochimie 20030801 |
| Transition metal complexes of buparvaquone as potent new antimalarial agents. 1. Synthesis, X-ray crystal-structures, electrochemistry and antimalarial activity against Plasmodium falciparum. | Journal of inorganic biochemistry 20030701 |
| Constitutive exclusion of Csk from Hck-positive membrane microdomains permits Src kinase-dependent proliferation of Theileria-transformed B lymphocytes. | Blood 20030301 |
| Hijacking of host cell IKK signalosomes by the transforming parasite Theileria. | Science (New York, N.Y.) 20021101 |
| Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. | International journal of pharmaceutics 20020426 |
| Production and characterisation of mucoadhesive nanosuspensions for the formulation of bupravaquone. | International journal of pharmaceutics 20010219 |
Related Products
© 2019 Angene International Limited. All rights Reserved.