200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 86925-97-5
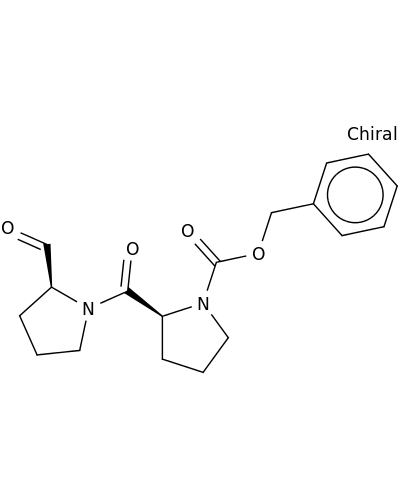
86925-97-5 | N-Benzyloxycarbonylprolylprolinal
CAS No: 86925-97-5 Catalog No: AG004OE6 MDL No:
Product Description
Catalog Number:
AG004OE6
Chemical Name:
N-Benzyloxycarbonylprolylprolinal
CAS Number:
86925-97-5
Molecular Formula:
C18H22N2O4
Molecular Weight:
330.3783
IUPAC Name:
benzyl (2S)-2-[(2S)-2-formylpyrrolidine-1-carbonyl]pyrrolidine-1-carboxylate
InChI:
InChI=1S/C18H22N2O4/c21-12-15-8-4-10-19(15)17(22)16-9-5-11-20(16)18(23)24-13-14-6-2-1-3-7-14/h1-3,6-7,12,15-16H,4-5,8-11,13H2/t15-,16-/m0/s1
InChI Key:
ORZXYSPOAVJYRU-HOTGVXAUSA-N
SMILES:
O=C[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)OCc1ccccc1
Properties
Complexity:
476
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
330.158g/mol
Formal Charge:
0
Heavy Atom Count:
24
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
330.384g/mol
Monoisotopic Mass:
330.158g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
66.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.7
Literature
| Title | Journal |
|---|---|
| Prolyl endopeptidase is involved in cellular signalling in human neuroblastoma SH-SY5Y cells. | Neuro-Signals 20110101 |
| Structural analysis of prolyl oligopeptidases using molecular docking and dynamics: insights into conformational changes and ligand binding. | PloS one 20110101 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Inhibition of prolyl oligopeptidase with a synthetic unnatural dipeptide. | Bioorganic & medicinal chemistry 20100701 |
| Inhibitors of prolyl oligopeptidases for the therapy of human diseases: defining diseases and inhibitors. | Journal of medicinal chemistry 20100513 |
| Molecular dynamics study of prolyl oligopeptidase with inhibitor in binding cavity. | SAR and QSAR in environmental research 20091001 |
| Intracellular activation and deactivation of tasidotin, an analog of dolastatin 15: correlation with cytotoxicity. | Molecular pharmacology 20090101 |
| Pharmacokinetics in mice implanted with xenografted tumors after intravenous administration of tasidotin (ILX651) or its carboxylate metabolite. | The AAPS journal 20070901 |
| A prolyl oligopeptidase inhibitor, Z-Pro-Prolinal, inhibits glyceraldehyde-3-phosphate dehydrogenase translocation and production of reactive oxygen species in CV1-P cells exposed to 6-hydroxydopamine. | Toxicology in vitro : an international journal published in association with BIBRA 20061201 |
| Dicarboxylic acid azacycle l-prolyl-pyrrolidine amides as prolyl oligopeptidase inhibitors and three-dimensional quantitative structure-activity relationship of the enzyme-inhibitor interactions. | Journal of medicinal chemistry 20050728 |
| Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. | Hypertension (Dallas, Tex. : 1979) 20040501 |
| Two peptidase activities decrease in treated bipolar disorder not schizophrenic patients. | Bipolar disorders 20040401 |
| New bioactive diterpene polyesters from Euphorbia decipiens. | Journal of natural products 20030901 |
| Dicarboxylic acid bis(L-prolyl-pyrrolidine) amides as prolyl oligopeptidase inhibitors. | Journal of medicinal chemistry 20020926 |
| A prolyl endopeptidase-inhibiting benzofuran dimer from Polyozellus multiflex. | Journal of natural products 20020101 |
| Purification and characterization of a Z-pro-prolinal-insensitive Z-Gly-Pro-7-amino-4-methyl coumarin-hydrolyzing peptidase from bovine serum--a new proline-specific peptidase. | Protein expression and purification 20010701 |
| New prolyl endopeptidase inhibitors: in vitro and in vivo activities of azabicyclo[2.2.2]octane, azabicyclo[2.2.1]heptane, and perhydroindole derivatives. | Journal of medicinal chemistry 19960607 |
Related Products
© 2019 Angene International Limited. All rights Reserved.