200,000+ products from a single source!
sales@angenechem.com
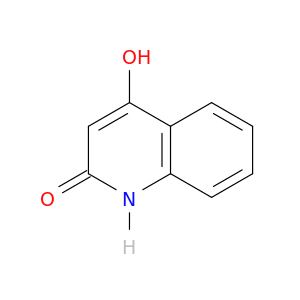
86-95-3 | 4-Hydroxyquinolin-2(1H);-one
CAS No: 86-95-3 Catalog No: AG004HLX MDL No:MFCD00277932
Product Description
Catalog Number:
AG004HLX
Chemical Name:
4-Hydroxyquinolin-2(1H);-one
CAS Number:
86-95-3
Molecular Formula:
C9H7NO2
Molecular Weight:
161.1574
MDL Number:
MFCD00277932
IUPAC Name:
4-hydroxy-1H-quinolin-2-one
InChI:
InChI=1S/C9H7NO2/c11-8-5-9(12)10-7-4-2-1-3-6(7)8/h1-5H,(H2,10,11,12)
InChI Key:
HDHQZCHIXUUSMK-UHFFFAOYSA-N
SMILES:
O=c1cc(O)c2c([nH]1)cccc2
EC Number:
201-711-0
UNII:
N58HX8G9CN
Properties
Complexity:
235
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
161.048g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
161.16g/mol
Monoisotopic Mass:
161.048g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
49.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.7
Literature
| Title | Journal |
|---|---|
| Design, synthesis and antitubercular potency of 4-hydroxyquinolin-2(1H)-ones. | European journal of medicinal chemistry 20170929 |
| Quorum sensing modulates colony morphology through alkyl quinolones in Pseudomonas aeruginosa. | BMC microbiology 20120101 |
| Honey's Ability to Counter Bacterial Infections Arises from Both Bactericidal Compounds and QS Inhibition. | Frontiers in microbiology 20120101 |
| Pseudomonas aeruginosa overrides the virulence inducing effect of opioids when it senses an abundance of phosphate. | PloS one 20120101 |
| Synthesis and biotransformation of 2-alkyl-4(1H)-quinolones by recombinant Pseudomonas putida KT2440. | Applied microbiology and biotechnology 20110901 |
| A quorum sensing regulated small volatile molecule reduces acute virulence and promotes chronic infection phenotypes. | PLoS pathogens 20110801 |
| Quinolones: from antibiotics to autoinducers. | FEMS microbiology reviews 20110301 |
| Electrochemically induced multicomponent assembling of isatins, 4-hydroxyquinolin-2(1H)-one and malononitrile: a convenient and efficient way to functionalized spirocyclic [indole-3,4'-pyrano[3,2-c]quinoline] scaffold. | Molecular diversity 20101101 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. | Environmental microbiology 20100601 |
| Homeostatic interplay between bacterial cell-cell signaling and iron in virulence. | PLoS pathogens 20100301 |
| Structure of PqsD, a Pseudomonas quinolone signal biosynthetic enzyme, in complex with anthranilate. | Biochemistry 20090915 |
| Development of N-[11C]methylamino 4-hydroxy-2(1H)-quinolone derivatives as PET radioligands for the glycine-binding site of NMDA receptors. | Bioorganic & medicinal chemistry 20090801 |
| Ring-substituted 4-hydroxy-1H-quinolin-2-ones: preparation and biological activity. | Molecules (Basel, Switzerland) 20090313 |
| Design and synthesis of 5-alkoxy-[1,2,4]triazolo[4,3-a]quinoline derivatives with anticonvulsant activity. | European journal of medicinal chemistry 20090301 |
| PqsD is responsible for the synthesis of 2,4-dihydroxyquinoline, an extracellular metabolite produced by Pseudomonas aeruginosa. | The Journal of biological chemistry 20081024 |
| Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. | PLoS pathogens 20081001 |
| A liquid chromatography-quadrupole time-of-flight (LC-QTOF)-based metabolomic approach reveals new metabolic effects of catechin in rats fed high-fat diets. | Journal of proteome research 20080601 |
| PqsA is required for the biosynthesis of 2,4-dihydroxyquinoline (DHQ), a newly identified metabolite produced by Pseudomonas aeruginosa and Burkholderia thailandensis. | Biological chemistry 20070801 |
| Microwave prompted multigram synthesis, structural determination, and photo-antiproliferative activity of fluorinated 4-hydroxyquinolinones. | Bioorganic & medicinal chemistry letters 20070101 |
| Studies on parasitologic and haematologic activities of an enaminone derivative of 4-hydroxyquinolin-2(1H)-one against murine schistosomiasis mansoni. | MedGenMed : Medscape general medicine 20070101 |
| Diversity-oriented synthesis of functionalized quinolin-2(1H)-ones via Pd-catalyzed site-selective cross-coupling reactions. | Journal of combinatorial chemistry 20070101 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Synthesis and SAR of highly potent and selective dopamine D(3)-receptor antagonists: Quinolin(di)one and benzazepin(di)one derivatives. herve.geneste@abbott.com. | Bioorganic & medicinal chemistry letters 20060201 |
| Synthesis of 4-hydroxycoumarin and 2,4-quinolinediol derivatives and evaluation of their effects on the viability of HepG2 cells and human hepatocytes culture. | European journal of medicinal chemistry 20041101 |
| CoMFA and homology-based models of the glycine binding site of N-methyl-d-aspartate receptor. | Journal of medicinal chemistry 20030424 |
| Synthesis and antibacterial activity of 2-substituted 6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acids. | Farmaco (Societa chimica italiana : 1989) 20010901 |
| 4-Hydroxy-6-oxo-6,7-dihydro-thieno[2,3-b] pyrimidine derivatives: synthesis and their biological evaluation for the glycine site acting on the N-methyl-D-aspartate (NMDA) receptor. | Archives of pharmacal research 20010801 |
Related Products
© 2019 Angene International Limited. All rights Reserved.