200,000+ products from a single source!
sales@angenechem.com
Home > Boronic Acids > 86-58-8
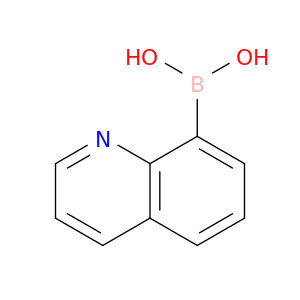
86-58-8 | Quinolin-8-ylboronic acid
CAS No: 86-58-8 Catalog No: AG003U3O MDL No:MFCD01114698
Product Description
Catalog Number:
AG003U3O
Chemical Name:
Quinolin-8-ylboronic acid
CAS Number:
86-58-8
Molecular Formula:
C9H8BNO2
Molecular Weight:
172.9763
MDL Number:
MFCD01114698
IUPAC Name:
quinolin-8-ylboronic acid
InChI:
InChI=1S/C9H8BNO2/c12-10(13)8-5-1-3-7-4-2-6-11-9(7)8/h1-6,12-13H
InChI Key:
KXJJSKYICDAICD-UHFFFAOYSA-N
SMILES:
OB(c1cccc2c1nccc2)O
UNII:
5QS1A25IJ6
Properties
Complexity:
177
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
173.065g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
172.978g/mol
Monoisotopic Mass:
173.065g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
53.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Reactivity of the bifunctional ambiphilic molecule 8-(dimesitylboryl)quinoline: hydrolysis and coordination to Cu(I), Ag(I) and Pd(II). | Dalton transactions (Cambridge, England : 2003) 20101207 |
| Determination of trace alkaline phosphatase by affinity adsorption solid substrate room temperature phosphorimetry based on wheat germ agglutinin labeled with 8-quinolineboronic acid phosphorescent molecular switch and prediction of diseases. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20100901 |
| 8-Quinolineboronic acid as a potential phosphorescent molecular switch for the determination of alpha-fetoprotein variant for the prediction of primary hepatocellular carcinoma. | Analytica chimica acta 20100324 |
| Synthesis of aminoboronic acids and their applications in bifunctional catalysis. | Accounts of chemical research 20090616 |
| A novel type of fluorescent boronic acid that shows large fluorescence intensity changes upon binding with a carbohydrate in aqueous solution at physiological pH. | Bioorganic & medicinal chemistry letters 20030324 |
Related Products
© 2019 Angene International Limited. All rights Reserved.