200,000+ products from a single source!
sales@angenechem.com
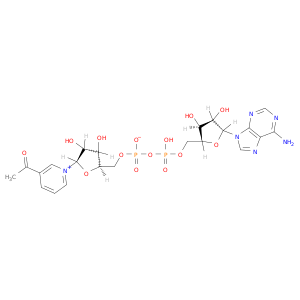
86-08-8 | Adenosine 5'-(trihydrogen diphosphate), P'?5'-ester with3-acetyl-1-b-D-ribofuranosylpyridinium, inner salt
CAS No: 86-08-8 Catalog No: AG00GRCE MDL No:
Product Description
Catalog Number:
AG00GRCE
Chemical Name:
Adenosine 5'-(trihydrogen diphosphate), P'?5'-ester with3-acetyl-1-b-D-ribofuranosylpyridinium, inner salt
CAS Number:
86-08-8
Molecular Formula:
C22H28N6O14P2
Molecular Weight:
662.4370
IUPAC Name:
[(2R,3S,4R,5R)-5-(3-acetylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] phosphate
InChI:
InChI=1S/C22H28N6O14P2/c1-10(29)11-3-2-4-27(5-11)21-17(32)15(30)12(40-21)6-38-43(34,35)42-44(36,37)39-7-13-16(31)18(33)22(41-13)28-9-26-14-19(23)24-8-25-20(14)28/h2-5,8-9,12-13,15-18,21-22,30-33H,6-7H2,1H3,(H3-,23,24,25,34,35,36,37)/t12-,13-,15-,16-,17-,18-,21-,22-/m1/s1
InChI Key:
KPVQNXLUPNWQHM-RBEMOOQDSA-N
SMILES:
O[C@@H]1[C@@H](COP(=O)(OP(=O)(OC[C@H]2O[C@H]([C@@H]([C@@H]2O)O)n2cnc3c2ncnc3N)O)[O-])O[C@H]([C@@H]1O)[n+]1cccc(c1)C(=O)C
EC Number:
201-649-4
Properties
Complexity:
1120
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
8
Defined Bond Stereocenter Count:
0
Exact Mass:
662.114g/mol
Formal Charge:
0
Heavy Atom Count:
44
Hydrogen Bond Acceptor Count:
18
Hydrogen Bond Donor Count:
6
Isotope Atom Count:
0
Molecular Weight:
662.442g/mol
Monoisotopic Mass:
662.114g/mol
Rotatable Bond Count:
11
Topological Polar Surface Area:
295A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-5.2
Literature
| Title | Journal |
|---|---|
| Reactions of the flavin mononucleotide in complex I: a combined mechanism describes NADH oxidation coupled to the reduction of APAD+, ferricyanide, or molecular oxygen. | Biochemistry 20091222 |
| A kinetic alignment of orthologous inosine-5'-monophosphate dehydrogenases. | Biochemistry 20080819 |
| Transhydrogenation reactions catalyzed by mitochondrial NADH-ubiquinone oxidoreductase (Complex I). | Biochemistry 20071211 |
| Structure of lactate dehydrogenase from Plasmodium vivax: complexes with NADH and APADH. | Biochemistry 20051213 |
| Structure of Toxoplasma gondii LDH1: active-site differences from human lactate dehydrogenases and the structural basis for efficient APAD+ use. | Biochemistry 20040203 |
| Kinetics of the transhydrogenase reaction catalyzed by mitochondrial NADH:ubiquinone oxidoreductase (complex I). | Biochemistry. Biokhimiia 20020601 |
| Substrate specificity of mammalian pyridine nucleotide transglycosidases. | Journal of nutritional science and vitaminology 20020601 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.