200,000+ products from a single source!
sales@angenechem.com
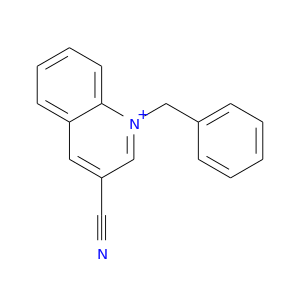
85289-84-5 | Quinolinium, 3-cyano-1-(phenylmethyl)-
CAS No: 85289-84-5 Catalog No: AG004LSE MDL No:
Product Description
Catalog Number:
AG004LSE
Chemical Name:
Quinolinium, 3-cyano-1-(phenylmethyl)-
CAS Number:
85289-84-5
Molecular Formula:
C17H13N2+
Molecular Weight:
245.2985
IUPAC Name:
1-benzylquinolin-1-ium-3-carbonitrile
InChI:
InChI=1S/C17H13N2/c18-11-15-10-16-8-4-5-9-17(16)19(13-15)12-14-6-2-1-3-7-14/h1-10,13H,12H2/q+1
InChI Key:
RAWCJBNTWIPXSE-UHFFFAOYSA-N
SMILES:
N#Cc1cc2ccccc2[n+](c1)Cc1ccccc1
Properties
Complexity:
336
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
245.108g/mol
Formal Charge:
1
Heavy Atom Count:
19
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
245.305g/mol
Monoisotopic Mass:
245.108g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
27.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.6
Literature
| Title | Journal |
|---|---|
| Oxygen-initiated chain mechanism for hydride transfer between NADH and NAD+ models. Reaction of 1-benzyl-3-cyanoquinolinium ion with N-methyl-9,10-dihydroacridine in acetonitrile. | The Journal of organic chemistry 20121019 |
| A new automated method to assess the rat recognition memory: validation of the method. | Behavioural brain research 20110912 |
| Intramolecular kinetic isotope effect in hydride transfer from dihydroacridine to a quinolinium ion. Rejection of a proposed two-step mechanism with a kinetically significant intermediate. | Organic & biomolecular chemistry 20080921 |
| Hydride-exchange reactions between NADH and NAD+ model compounds under non-steady-state conditions. Apparent and real kinetic isotope effects. | Organic & biomolecular chemistry 20030107 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







