200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 84-60-6
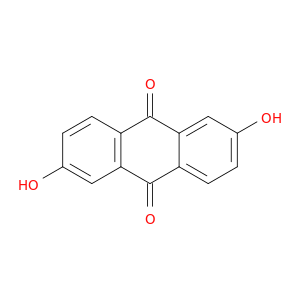
84-60-6 | 2,6-Dihydroxyanthracene-9,10-dione
CAS No: 84-60-6 Catalog No: AG00331R MDL No:MFCD00001228
Product Description
Catalog Number:
AG00331R
Chemical Name:
2,6-Dihydroxyanthracene-9,10-dione
CAS Number:
84-60-6
Molecular Formula:
C14H8O4
Molecular Weight:
240.2109
MDL Number:
MFCD00001228
IUPAC Name:
2,6-dihydroxyanthracene-9,10-dione
InChI:
InChI=1S/C14H8O4/c15-7-1-3-9-11(5-7)14(18)10-4-2-8(16)6-12(10)13(9)17/h1-6,15-16H
InChI Key:
APAJFZPFBHMFQR-UHFFFAOYSA-N
SMILES:
Oc1ccc2c(c1)C(=O)c1c(C2=O)cc(cc1)O
EC Number:
201-544-3
UNII:
W83883330W
NSC Number:
33531
Properties
Complexity:
342
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
240.042g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
240.214g/mol
Monoisotopic Mass:
240.042g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
74.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.2
Literature
| Title | Journal |
|---|---|
| Determination of the optical GAP in thin films of amorphous dilithium phthalocyanine using the Tauc and Cody models. | Molecules (Basel, Switzerland) 20120824 |
| Phenol transformation photosensitised by quinoid compounds. | Physical chemistry chemical physics : PCCP 20110621 |
| Correlation between bilirubin glucuronidation and estradiol-3-gluronidation in the presence of model UDP-glucuronosyltransferase 1A1 substrates/inhibitors. | Drug metabolism and disposition: the biological fate of chemicals 20110201 |
| Electrical and optical properties of copper and nickel molecular materials with tetrabenzo [b,f,j,n] [1,5,9,13] tetraazacyclohexadecine thin films grown by the vacuum thermal evaporation technique. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20100101 |
| Structure-activity relationships of anthraquinones on the suppression of DNA-binding activity of the aryl hydrocarbon receptor induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. | Journal of bioscience and bioengineering 20090301 |
| Correlation between reduction potentials and inhibitions of Epstein-Barr virus activation by anthraquinone derivatives. | Bioorganic & medicinal chemistry letters 20080715 |
| Synthesis and anti-inflammatory activity of 3-(4'-geranyloxy-3'-methoxyphenyl)-2-trans propenoic acid and its ester derivatives. | Bioorganic & medicinal chemistry letters 20071015 |
| Interaction of anthracene and its oxidative derivatives with human serum albumin. | Acta biochimica Polonica 20060101 |
| Structure and kinetics of a transient antibody binding intermediate reveal a kinetic discrimination mechanism in antigen recognition. | Proceedings of the National Academy of Sciences of the United States of America 20050906 |
| Differential and special properties of the major human UGT1-encoded gastrointestinal UDP-glucuronosyltransferases enhance potential to control chemical uptake. | The Journal of biological chemistry 20040109 |
| The specificity of cross-reactivity: promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. | Protein science : a publication of the Protein Society 20031001 |
| The effect of incubation conditions on the enzyme kinetics of udp-glucuronosyltransferases. | Drug metabolism and disposition: the biological fate of chemicals 20030601 |
| Evidence for phosphorylation requirement for human bilirubin UDP-glucuronosyltransferase (UGT1A1) activity. | Biochemical and biophysical research communications 20030328 |
| Inhibition and active sites of UDP-glucuronosyltransferases 2B7 and 1A1. | Drug metabolism and disposition: the biological fate of chemicals 20021201 |
| Differential modulation of UDP-glucuronosyltransferase 1A1 (UGT1A1)-catalyzed estradiol-3-glucuronidation by the addition of UGT1A1 substrates and other compounds to human liver microsomes. | Drug metabolism and disposition: the biological fate of chemicals 20021101 |
| Biosynthesis of drug glucuronides for use as authentic standards. | Journal of pharmacological and toxicological methods 20020101 |
| Induction of cytochromes P450 1A1 and 1B1 by emodin in human lung adenocarcinoma cell line CL5. | Drug metabolism and disposition: the biological fate of chemicals 20010901 |
| Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): structure-requirement of hydroxyanthraquinones for estrogenic activity. | Bioorganic & medicinal chemistry letters 20010723 |
| Hydroxyquinones are competitive non-peptide inhibitors of HIV-1 proteinase. | Biochimica et biophysica acta 19951115 |
| Stable expression of a human liver UDP-glucuronosyltransferase (UGT2B15) with activity toward steroid and xenobiotic substrates. | Drug metabolism and disposition: the biological fate of chemicals 19940101 |
| Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinone derivatives against human cytomegalovirus. | Antiviral research 19920101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.