200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 83-31-8
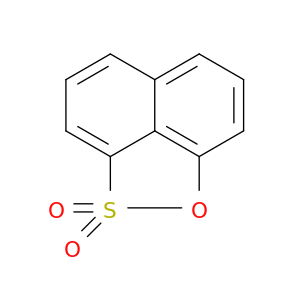
83-31-8 | Naphtho[1,8-cd][1,2]oxathiole 2,2-dioxide
CAS No: 83-31-8 Catalog No: AG003DS9 MDL No:MFCD00005937
Product Description
Catalog Number:
AG003DS9
Chemical Name:
Naphtho[1,8-cd][1,2]oxathiole 2,2-dioxide
CAS Number:
83-31-8
Molecular Formula:
C10H6O3S
Molecular Weight:
206.2178
MDL Number:
MFCD00005937
IUPAC Name:
2-oxa-3λ6-thiatricyclo[6.3.1.04,12]dodeca-1(11),4,6,8(12),9-pentaene 3,3-dioxide
InChI:
InChI=1S/C10H6O3S/c11-14(12)9-6-2-4-7-3-1-5-8(13-14)10(7)9/h1-6H
InChI Key:
IEIADDVJUYQKAZ-UHFFFAOYSA-N
SMILES:
O=S1(=O)Oc2c3c1cccc3ccc2
EC Number:
201-468-0
NSC Number:
26341
Properties
Complexity:
330
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
206.004g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
206.215g/mol
Monoisotopic Mass:
206.004g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
51.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.3
Literature
| Title | Journal |
|---|---|
| Synthesis and reactivity of a bis-sultone cross-linker for peptide conjugation and [18F]-radiolabelling via unusual 'double click' approach. | Organic & biomolecular chemistry 20120207 |
| Sultone opening with [18F]fluoride: an efficient 18F-labelling strategy for PET imaging. | Chemical communications (Cambridge, England) 20111107 |
| Isolation of a sultone as an unusual acetolysis product. | Carbohydrate research 20110501 |
| 4-Benzyloxy-gamma-sultone derivatives: discovery of a novel family of non-nucleoside inhibitors of human cytomegalovirus and varicella zoster virus. | Journal of medicinal chemistry 20090326 |
| Chloroalkylsulfonate ionic liquids by ring opening of sultones with organic chloride salts. | Chemical communications (Cambridge, England) 20080907 |
| Delta-sultone formation through Rh-catalyzed C-H insertion. | Organic letters 20071011 |
| Studies of the chemical selectivity of hapten, reactivity, and skin sensitization potency. 1. Synthesis and studies on the reactivity toward model nucleophiles of the (13)C-labeled skin sensitizers hex-1-ene- and hexane-1,3-sultones. | Chemical research in toxicology 20010101 |
| Studies of the chemical selectivity of hapten, reactivity, and skin sensitization potency. 2. nmr studies of the covalent binding of the (13)c-labeled skin sensitizers 2-[13C]- and 3-[13C]hex-1-ene- and 3-[13C]hexane-1,3-sultones to human serum albumin. | Chemical research in toxicology 20010101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







