200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 827-54-3
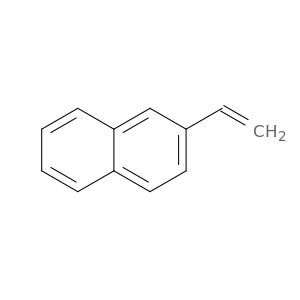
827-54-3 | 2-Vinylnaphthalene
CAS No: 827-54-3 Catalog No: AG0038EV MDL No:MFCD00004125
Product Description
Catalog Number:
AG0038EV
Chemical Name:
2-Vinylnaphthalene
CAS Number:
827-54-3
Molecular Formula:
C12H10
Molecular Weight:
154.2078
MDL Number:
MFCD00004125
IUPAC Name:
2-ethenylnaphthalene
InChI:
InChI=1S/C12H10/c1-2-10-7-8-11-5-3-4-6-12(11)9-10/h2-9H,1H2
InChI Key:
KXYAVSFOJVUIHT-UHFFFAOYSA-N
SMILES:
C=Cc1ccc2c(c1)cccc2
EC Number:
212-573-6
UNII:
HZD8LI91N1
NSC Number:
177870
Properties
Complexity:
159
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
154.078g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
154.212g/mol
Monoisotopic Mass:
154.078g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
0A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.3
Literature
| Title | Journal |
|---|---|
| 2-Bromo-1,3-bis[2-(2-naphthyl)vinyl]benzene benzene hemisolvate and 9-bromodinaphth[1,2-a:2',1'-j]anthracene. | Acta crystallographica. Section C, Crystal structure communications 20110101 |
| Synthesis and biological evaluation of arylidene analogues of Meldrum's acid as a new class of antimalarial and antioxidant agents. | Bioorganic & medicinal chemistry 20100801 |
| Expanding the useful range of ionic liquids: melting point depression of organic salts with carbon dioxide for biphasic catalytic reactions. | Chemical communications (Cambridge, England) 20060921 |
| Preparation of a novel fluorescence nanoparticles and its application in the determination of Hg(II). | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20051101 |
| Intermolecular, markovnikov hydroamination of vinylarenes with alkylamines. | Journal of the American Chemical Society 20031126 |
| Metalloporphyrin-mediated asymmetric nitrogen-atom transfer to hydrocarbons: aziridination of alkenes and amidation of saturated C-H bonds catalyzed by chiral ruthenium and manganese porphyrins. | Chemistry (Weinheim an der Bergstrasse, Germany) 20020402 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.