200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 82586-52-5
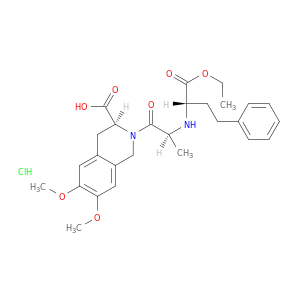
82586-52-5 | Moexipril hydrochloride
CAS No: 82586-52-5 Catalog No: AG004XN5 MDL No:MFCD00940481
Product Description
Catalog Number:
AG004XN5
Chemical Name:
Moexipril hydrochloride
CAS Number:
82586-52-5
Molecular Formula:
C27H35ClN2O7
Molecular Weight:
535.0290
MDL Number:
MFCD00940481
IUPAC Name:
(3S)-2-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-6,7-dimethoxy-3,4-dihydro-1H-isoquinoline-3-carboxylic acid;hydrochloride
InChI:
InChI=1S/C27H34N2O7.ClH/c1-5-36-27(33)21(12-11-18-9-7-6-8-10-18)28-17(2)25(30)29-16-20-15-24(35-4)23(34-3)14-19(20)13-22(29)26(31)32;/h6-10,14-15,17,21-22,28H,5,11-13,16H2,1-4H3,(H,31,32);1H/t17-,21-,22-;/m0./s1
InChI Key:
JXRAXHBVZQZSIC-JKVLGAQCSA-N
SMILES:
CCOC(=O)[C@@H](N[C@H](C(=O)N1Cc2cc(OC)c(cc2C[C@H]1C(=O)O)OC)C)CCc1ccccc1.Cl
UNII:
Q1UMG3UH45
Properties
Complexity:
742
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
2
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
534.213g/mol
Formal Charge:
0
Heavy Atom Count:
37
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
535.034g/mol
Monoisotopic Mass:
534.213g/mol
Rotatable Bond Count:
12
Topological Polar Surface Area:
114A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| A rapid and sensitive liquid chromatography-tandem mass spectrometric assay for moexipril, an angiotensin-converting enzyme inhibitor in human plasma. | Biomedical chromatography : BMC 20121201 |
| Spectrofluorimetric determination of etodolac, moxepril HCl and fexofenadine HCl using europium sensitized fluorescence in bulk and pharmaceutical preparations. | Journal of fluorescence 20120101 |
| First order derivative spectrophotometric and HPLC methods for determination of moexipril hydrochloride in the pure form, pharmeceutical formulations and evaluation of its stability. | Acta poloniae pharmaceutica 20120101 |
| Determination of antihypertensive drug moexipril hydrochloride based on the enhancement effect of sodium dodecyl sulfate at carbon paste electrode. | Talanta 20100415 |
| Moexipril for treatment of primary biliary cirrhosis in patients with an incomplete response to ursodeoxycholic acid. | Digestive diseases and sciences 20100201 |
| [Preventive pharmacotherapy in arterial hypertension: problems of clinical assessment of drugs in women]. | Kardiologiia 20090101 |
| Moexipril and left ventricular hypertrophy. | Vascular health and risk management 20070201 |
| Metabolism of moexipril to moexiprilat: determination of in vitro metabolism using HPLC-ES-MS. | Medicinal chemistry (Shariqah (United Arab Emirates)) 20070101 |
| [Assessment of the effect of angiotensin converting enzyme inhibitor moexipril on the functional state of vascular wall in patients with I - III degree arterial hypertension]. | Kardiologiia 20070101 |
| Monitoring the metabolism of moexipril to moexiprilat using high-performance liquid chromatography-electrospray ionization mass spectrometry. | Journal of chromatographic science 20060401 |
| [Comparative efficacy and safety of contemporary Angiotensin converting enzyme inhibitors moexipril and spirapril in women with postmenopausal metabolic syndrome]. | Kardiologiia 20060101 |
| [Investigation of efficacy of cardiovascular drugs in women]. | Kardiologiia 20060101 |
| [Hypotensive, organoprotective, and metabolic effects of Angiotensin converting enzyme inhibitor moexipril in women with postmenopausal syndrome]. | Kardiologiia 20060101 |
| [Moexipril and cardiovascular diseases in women: is there a reason for optimism?]. | Kardiologiia 20060101 |
| [Dynamics of left ventricular longitudinal function in patients with arterial hypertension during therapy with angiotensin converting enzyme inhibitor moexipril]. | Kardiologiia 20060101 |
| [Angiotensin converting enzyme inhibitor moexipril in the treatment of arterial hypertension. Possibilities of moexipril in the treatment of postmenopausal women]. | Kardiologiia 20060101 |
| Subchronic exposure to high-dose ACE-inhibitor moexipril induces catalase activity in rat liver. | Molecular and cellular biochemistry 20051201 |
| MORE--MOexipril and REgression of left ventricle hypertrophy in combination therapy A multicentric open label clinical trial. | International journal of cardiology 20050420 |
| Regression of left ventricular hypertrophy with moexipril, an angiotensin-converting enzyme inhibitor, in hypertensive patients. | American journal of therapeutics 20050101 |
| [Hemodynamic and anti-ischemic effects of moexipril in patients having postinfarction heart dysfunction and moderate left ventricular heart failure]. | Klinicheskaia meditsina 20050101 |
| [The use of moexipril in postmenopausal women with hypertension and associated changes of bone mineral density]. | Kardiologiia 20050101 |
| [Moexipril influence on quality of life in postmenopausal women with arterial hypertension]. | Terapevticheskii arkhiv 20050101 |
| [Assessment of antihypertensive efficacy of moexipril in metabolic syndrome]. | Kardiologiia 20050101 |
| Pharmacological and clinical profile of moexipril: a concise review. | Journal of clinical pharmacology 20040801 |
| The influence of relative humidity and temperature on stability of moexipril hydrochloride in solid phase. | Acta poloniae pharmaceutica 20040101 |
| Simultaneous determination of moexipril hydrochloride and hydrochlorothiazide in tablets by derivative spectrophotometric and high-performance liquid chromatographic methods. | Journal of pharmaceutical and biomedical analysis 20031015 |
| Pharmacological profile and clinical use of moexipril. | Expert review of cardiovascular therapy 20030901 |
| Moexipril and quinapril inhibition of tissue angiotensin-converting enzyme activity in the rat: evidence for direct effects in heart, lung and kidney and stimulation of prostacyclin generation. | Journal of endocrinological investigation 20030101 |
| ACE Inhibition with moexipril: a review of potential effects beyond blood pressure control. | American journal of cardiovascular drugs : drugs, devices, and other interventions 20030101 |
| Using ACE inhibitors appropriately. | American family physician 20020801 |
| Oestrogen action on the myocardium in vivo: specific and permissive for angiotensin-converting enzyme inhibition. | Journal of hypertension 20020501 |
| Should perindopril be used to treat patients with heart failure? | American family physician 20020501 |
| Reversal of left ventricular hypertrophy with the ACE inhibitor moexipril in patients with essential hypertension. | Acta cardiologica 20020201 |
| Moexipril shows a long duration of action related to an extended pharmacokinetic half-life and prolonged ACE inhibition. | International journal of clinical pharmacology and therapeutics 20020101 |
| Enalapril and moexipril protect from free radical-induced neuronal damage in vitro and reduce ischemic brain injury in mice and rats. | European journal of pharmacology 19990528 |
| Increased arterial distensibility in postmenopausal hypertensive women with and without hormone replacement therapy after acute administration of the ACE inhibitor moexipril. | Cardiovascular drugs and therapy 19980901 |
| Moexipril. A review of its use in the management of essential hypertension. | Drugs 19980601 |
| Antihypertensive treatment in postmenopausal women: results from a prospective, randomized, double-blind, controlled study comparing an ACE inhibitor (moexipril) with a diuretic (hydrochlorothiazide). | Cardiology 19980501 |
| Impact of antihypertensive therapy on the skeleton: effects of moexipril and hydrochlorothiazide on osteopenia in spontaneously hypertensive ovariectomized rats. | The Journal of endocrinology 19970901 |
| Pharmacological and toxicological studies of the new angiotensin converting enzyme inhibitor moexipril hydrochloride. | Arzneimittel-Forschung 19970201 |
| Evaluation of the antihypertensive efficacy and tolerability of moexipril, a new ACE inhibitor, compared to hydrochlorothiazide in elderly patients. | European journal of clinical pharmacology 19960101 |
| Tricenter assessment of the efficacy of the ACE inhibitor, moexipril, by ambulatory blood pressure monitoring. | Journal of clinical pharmacology 19950301 |
| Evidence for site-specific absorption of a novel ACE inhibitor. | Pharmaceutical research 19890901 |
Related Products
© 2019 Angene International Limited. All rights Reserved.