200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 818-72-4
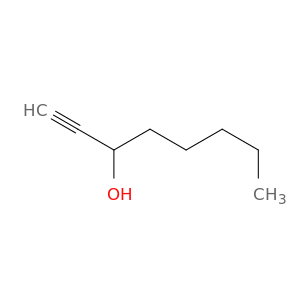
818-72-4 | Oct-1-yn-3-ol
CAS No: 818-72-4 Catalog No: AG003EL1 MDL No:MFCD00004588
Product Description
Catalog Number:
AG003EL1
Chemical Name:
Oct-1-yn-3-ol
CAS Number:
818-72-4
Molecular Formula:
C8H14O
Molecular Weight:
126.1962
MDL Number:
MFCD00004588
IUPAC Name:
oct-1-yn-3-ol
InChI:
InChI=1S/C8H14O/c1-3-5-6-7-8(9)4-2/h2,8-9H,3,5-7H2,1H3
InChI Key:
VUGRNZHKYVHZSN-UHFFFAOYSA-N
SMILES:
CCCCCC(C#C)O
EC Number:
212-455-4
Properties
Complexity:
98.4
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
126.104g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
126.199g/mol
Monoisotopic Mass:
126.104g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
20.2A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
2
Literature
| Title | Journal |
|---|---|
| Functional characterization of the octenol receptor neuron on the maxillary palps of the yellow fever mosquito, Aedes aegypti. | PloS one 20110101 |
| Characterization of an enantioselective odorant receptor in the yellow fever mosquito Aedes aegypti. | PloS one 20090101 |
| Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. | PloS one 20080101 |
| Evaluation of the enantiomers of 1-octen-3-ol and 1-octyn-3-ol as attractants for mosquitoes associated with a freshwater swamp in Florida, U.S.A. | Medical and veterinary entomology 20071201 |
| A straightforward synthesis of (-)-phaseolinic acid. | The Journal of organic chemistry 20041112 |
| Novel chemoenzymatic strategy for the synthesis of enantiomerically pure secondary alcohols with sterically similar substituents. | The Journal of organic chemistry 20030627 |
| Practical enantioresolution of alcohols with 2-methoxy-2-(1-naphthyl)propionic acid and determination of their absolute configurations by the (1)H NMR anisotropy method. | Chirality 20020101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.