200,000+ products from a single source!
sales@angenechem.com
Home > Nitro Compounds > 79917-37-6
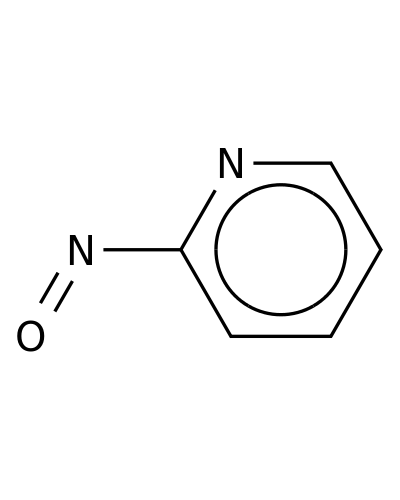
79917-37-6 | Pyridine, 2-nitroso-
CAS No: 79917-37-6 Catalog No: AG004Z9J MDL No:
Product Description
Catalog Number:
AG004Z9J
Chemical Name:
Pyridine, 2-nitroso-
CAS Number:
79917-37-6
Molecular Formula:
C5H4N2O
Molecular Weight:
108.0981
IUPAC Name:
2-nitrosopyridine
InChI:
InChI=1S/C5H4N2O/c8-7-5-3-1-2-4-6-5/h1-4H
InChI Key:
UJMHYOGYJDQSOH-UHFFFAOYSA-N
SMILES:
O=Nc1ccccn1
Properties
Complexity:
84.5
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
108.032g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
108.1g/mol
Monoisotopic Mass:
108.032g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
42.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.6
Literature
| Title | Journal |
|---|---|
| An allosteric modulator to control endogenous G protein-coupled receptors with light. | Nature chemical biology 20141001 |
| Lewis acid-catalyzed Friedel-Crafts alkylations of 3-hydroxy-2-oxindole: an efficient approach to the core structure of azonazine. | Chemical communications (Cambridge, England) 20121018 |
| Formation of isoxazolidines by enantioselective copper-catalyzed annulation of 2-nitrosopyridine with allylstannanes. | Angewandte Chemie (International ed. in English) 20111118 |
| Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola. | Organic letters 20101015 |
| Enantioselective nitroso-Diels-Alder reaction and its application for the synthesis of (-)-peracetylated conduramine A-1. | Chemistry (Weinheim an der Bergstrasse, Germany) 20090914 |
| Effect of para-sulfonato-calix[n]arenes on the solubility, chemical stability, and bioavailability of a water insoluble drug nifedipine. | Current drug discovery technologies 20080601 |
| Retinal penetration of calpain inhibitors in rats after oral administration. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20061201 |
| Rich chemistry of nitroso compounds. | Chemical communications (Cambridge, England) 20050728 |
| Catalytic, highly enantio, and diastereoselective nitroso diels-alder reaction. | Journal of the American Chemical Society 20040407 |
| Electrochemical and spectroelectrochemical behavior of the main photodegradation product of nifedipine: the nitrosopyridine derivative. | Pharmaceutical research 20020401 |
Related Products
© 2019 Angene International Limited. All rights Reserved.