200,000+ products from a single source!
sales@angenechem.com
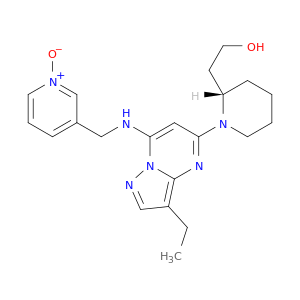
779353-01-4 | (2S)-1-[3-Ethyl-7-[[(1-oxido-3-pyridinyl)methyl]amino]pyrazolo[1,5-a]pyrimidin-5-yl]-2-piperidineethanol
CAS No: 779353-01-4 Catalog No: AG003AK1 MDL No:MFCD16037702
Product Description
Catalog Number:
AG003AK1
Chemical Name:
(2S)-1-[3-Ethyl-7-[[(1-oxido-3-pyridinyl)methyl]amino]pyrazolo[1,5-a]pyrimidin-5-yl]-2-piperidineethanol
CAS Number:
779353-01-4
Molecular Formula:
C21H28N6O2
Molecular Weight:
396.4860
MDL Number:
MFCD16037702
IUPAC Name:
2-[(2S)-1-[3-ethyl-7-[(1-oxidopyridin-1-ium-3-yl)methylamino]pyrazolo[1,5-a]pyrimidin-5-yl]piperidin-2-yl]ethanol
InChI:
InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1
InChI Key:
PIMQWRZWLQKKBJ-SFHVURJKSA-N
SMILES:
OCC[C@@H]1CCCCN1c1cc(NCc2ccc[n+](c2)[O-])n2c(n1)c(CC)cn2
UNII:
4V8ECV0NBQ
NSC Number:
747135
Properties
Complexity:
512
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
396.227g/mol
Formal Charge:
0
Heavy Atom Count:
29
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
396.495g/mol
Monoisotopic Mass:
396.227g/mol
Rotatable Bond Count:
7
Topological Polar Surface Area:
91.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.9
Literature
| Title | Journal |
|---|---|
| Aldo-keto reductase 1C3 (AKR1C3): a missing piece of the puzzle in the dinaciclib interaction profile. | Archives of toxicology 20180710 |
| CDK12 Inhibition Reverses De Novo and Acquired PARP Inhibitor Resistance in BRCA Wild-Type and Mutated Models of Triple-Negative Breast Cancer. | Cell reports 20161122 |
| Multiple CDK inhibitor dinaciclib suppresses neuroblastoma growth via inhibiting CDK2 and CDK9 activity. | Scientific reports 20160101 |
| Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains. | ACS chemical biology 20131115 |
| A CDK4/6 inhibitor enhances cytotoxicity of paclitaxel in lung adenocarcinoma cells harboring mutant KRAS as well as wild-type KRAS. | Cancer biology & therapy 20130701 |
| The requirement for cyclin D function in tumor maintenance. | Cancer cell 20121016 |
| Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. | Cancer cell 20121016 |
| Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. | Cell cycle (Georgetown, Tex.) 20120715 |
| Cyclin-dependent kinase inhibitor Dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. | Cancer biology & therapy 20111001 |
| Vinblastine sensitizes leukemia cells to cyclin-dependent kinase inhibitors, inducing acute cell cycle phase-independent apoptosis. | Cancer biology & therapy 20110815 |
| Establishing the carbon skeleton of pharmaceutical agents using HSQC-ADEQUATE spectra. | Journal of pharmaceutical and biomedical analysis 20110715 |
| The cyclin-dependent kinase inhibitor SCH 727965 (dinacliclib) induces the apoptosis of osteosarcoma cells. | Molecular cancer therapeutics 20110601 |
| Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. | Molecular cancer therapeutics 20110401 |
| Expression analysis and molecular targeting of cyclin-dependent kinases in advanced melanoma. | Cell cycle (Georgetown, Tex.) 20110315 |
| Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. | Molecular cancer therapeutics 20100801 |
| A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. | Cancer cell 20100713 |
| The landscape of somatic copy-number alteration across human cancers. | Nature 20100218 |
Related Products
© 2019 Angene International Limited. All rights Reserved.