200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 7784-09-0
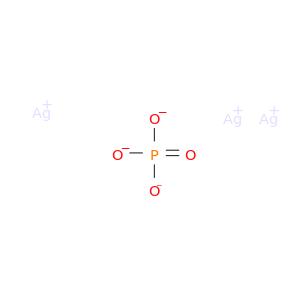
7784-09-0 | Trisilver phosphate
CAS No: 7784-09-0 Catalog No: AG0035L3 MDL No:MFCD00014148
Product Description
Catalog Number:
AG0035L3
Chemical Name:
Trisilver phosphate
CAS Number:
7784-09-0
Molecular Formula:
Ag3O4P
Molecular Weight:
418.5760
MDL Number:
MFCD00014148
IUPAC Name:
trisilver;phosphate
InChI:
InChI=1S/3Ag.H3O4P/c;;;1-5(2,3)4/h;;;(H3,1,2,3,4)/q3*+1;/p-3
InChI Key:
FJOLTQXXWSRAIX-UHFFFAOYSA-K
SMILES:
[O-]P(=O)([O-])[O-].[Ag+].[Ag+].[Ag+]
EC Number:
232-049-0
UNII:
ZL6T4Y1XP8
Properties
Complexity:
36.8
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
4
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
417.668g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
418.574g/mol
Monoisotopic Mass:
415.669g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
86.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Formal synthesis of (-)-cephalotaxine based on a tandem hydroamination/semipinacol rearrangement reaction. | Chemistry, an Asian journal 20120501 |
| Oxygen isotope analysis of phosphate: improved precision using TC/EA CF-IRMS. | Journal of mass spectrometry : JMS 20090601 |
| Toward general access to the aspidosperma-type terpenoid indole alkaloids: synthesis of the key 3,3-disubstituted piperidones through enantioselective intramolecular heck-type reaction of chloroformamides. | Chemical & pharmaceutical bulletin 20081101 |
| A powerful chiral counterion strategy for asymmetric transition metal catalysis. | Science (New York, N.Y.) 20070727 |
| Formation and reactivity of silacyclopropenes derived from siloxyalkynes: stereoselective formation of 1,2,4-triols. | Organic letters 20060831 |
Related Products
© 2019 Angene International Limited. All rights Reserved.