200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 766-55-2
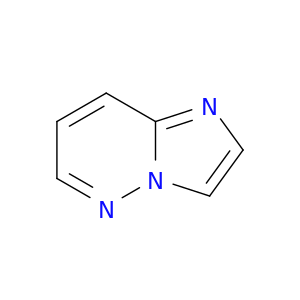
766-55-2 | Imidazo[1,2-b]pyridazine
CAS No: 766-55-2 Catalog No: AG003QZM MDL No:MFCD07782103
Product Description
Catalog Number:
AG003QZM
Chemical Name:
Imidazo[1,2-b]pyridazine
CAS Number:
766-55-2
Molecular Formula:
C6H5N3
Molecular Weight:
119.1240
MDL Number:
MFCD07782103
IUPAC Name:
imidazo[1,2-b]pyridazine
InChI:
InChI=1S/C6H5N3/c1-2-6-7-4-5-9(6)8-3-1/h1-5H
InChI Key:
VTVRXITWWZGKHV-UHFFFAOYSA-N
SMILES:
c1cnn2c(c1)ncc2
Properties
Complexity:
105
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
119.048g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
119.127g/mol
Monoisotopic Mass:
119.048g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
30.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.3
Literature
| Title | Journal |
|---|---|
| Design and synthesis of novel DFG-out RAF/vascular endothelial growth factor receptor 2 (VEGFR2) inhibitors. 1. Exploration of [5,6]-fused bicyclic scaffolds. | Journal of medicinal chemistry 20120412 |
| Discovery of imidazo[1,2-b]pyridazines as IKKβ inhibitors. Part 3: exploration of effective compounds in arthritis models. | Bioorganic & medicinal chemistry letters 20110801 |
| Mechanisms of cytotoxicity to Pim kinase inhibitor, SGI-1776, in acute myeloid leukemia. | Blood 20110721 |
| Biochemical characterization of TAK-593, a novel VEGFR/PDGFR inhibitor with a two-step slow binding mechanism. | Biochemistry 20110208 |
| Discovery of imidazo[1,2-b]pyridazines as IKKβ inhibitors. Part 2: improvement of potency in vitro and in vivo. | Bioorganic & medicinal chemistry letters 20110201 |
| Urocortin-1 within the centrally-projecting Edinger-Westphal nucleus is critical for ethanol preference. | PloS one 20110101 |
| Discovery of imidazo[1,2-b]pyridazine derivatives as IKKbeta inhibitors. Part 1: Hit-to-lead study and structure-activity relationship. | Bioorganic & medicinal chemistry letters 20100901 |
| Inhibitors of PIM-1 kinase: a computational analysis of the binding free energies of a range of imidazo [1,2-b] pyridazines. | Journal of chemical information and modeling 20100322 |
| Intra-periaqueductal grey microinjections of an imidazo[1,2-b]pyridazine derivative, DM2, affects rostral ventromedial medulla cell activity and shows antinociceptive effect. | Neuropharmacology 20100301 |
| Pim kinase inhibitor, SGI-1776, induces apoptosis in chronic lymphocytic leukemia cells. | Blood 20091105 |
| Kinase domain insertions define distinct roles of CLK kinases in SR protein phosphorylation. | Structure (London, England : 1993) 20090311 |
| T-type channel blocking properties and antiabsence activity of two imidazo[1,2-b]pyridazine derivatives structurally related to indomethacin. | Neuropharmacology 20090301 |
| Imidazo[1,2-b]pyridazines: a potent and selective class of cyclin-dependent kinase inhibitors. | Bioorganic & medicinal chemistry letters 20040503 |
Related Products
© 2019 Angene International Limited. All rights Reserved.