200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 763-35-9
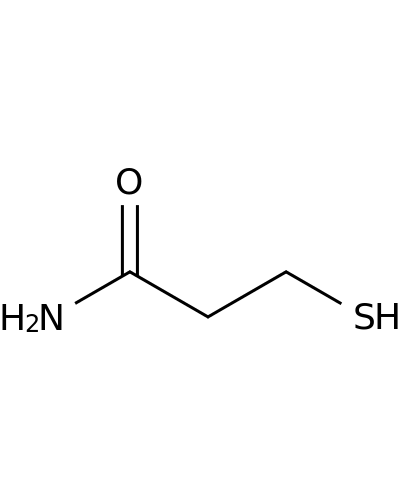
763-35-9 | 3-Mercaptopropionamide
CAS No: 763-35-9 Catalog No: AG0038FS MDL No:MFCD03005215
Product Description
Catalog Number:
AG0038FS
Chemical Name:
3-Mercaptopropionamide
CAS Number:
763-35-9
Molecular Formula:
C3H7NOS
Molecular Weight:
105.1588
MDL Number:
MFCD03005215
IUPAC Name:
3-sulfanylpropanamide
InChI:
InChI=1S/C3H7NOS/c4-3(5)1-2-6/h6H,1-2H2,(H2,4,5)
InChI Key:
JLSJEUQOXVVCPN-UHFFFAOYSA-N
SMILES:
NC(=O)CCS
Properties
Complexity:
54.8
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
105.025g/mol
Formal Charge:
0
Heavy Atom Count:
6
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
105.155g/mol
Monoisotopic Mass:
105.025g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
44.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.6
Literature
| Title | Journal |
|---|---|
| Direct catalytic enantio- and diastereoselective aldol reaction of thioamides. | Journal of the American Chemical Society 20110413 |
| Cell-penetrant, nanomolar O-GlcNAcase inhibitors selective against lysosomal hexosaminidases. | Chemistry & biology 20101124 |
| Thiolated quaternary ammonium-chitosan conjugates for enhanced precorneal retention, transcorneal permeation and intraocular absorption of dexamethasone. | European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 20100601 |
| Synthesis, characterization and evaluation of thiolated quaternary ammonium-chitosan conjugates for enhanced intestinal drug permeation. | European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 20090910 |
| Important role of the 3-mercaptopropionamide moiety in glutathione: promoting effect on decomposition of the adduct of glutathione with the oxoammonium ion of TEMPO. | The Journal of organic chemistry 20051014 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







