200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 76084-32-7
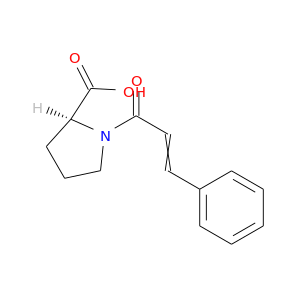
76084-32-7 | L-Proline, 1-(1-oxo-3-phenyl-2-propenyl)-
CAS No: 76084-32-7 Catalog No: AG005G7H MDL No:MFCD16377067
Product Description
Catalog Number:
AG005G7H
Chemical Name:
L-Proline, 1-(1-oxo-3-phenyl-2-propenyl)-
CAS Number:
76084-32-7
Molecular Formula:
C14H15NO3
Molecular Weight:
245.2738
MDL Number:
MFCD16377067
IUPAC Name:
(2S)-1-(3-phenylprop-2-enoyl)pyrrolidine-2-carboxylic acid
InChI:
InChI=1S/C14H15NO3/c16-13(9-8-11-5-2-1-3-6-11)15-10-4-7-12(15)14(17)18/h1-3,5-6,8-9,12H,4,7,10H2,(H,17,18)/t12-/m0/s1
InChI Key:
WSBOYOYQOJYXMU-LBPRGKRZSA-N
SMILES:
OC(=O)[C@@H]1CCCN1C(=O)C=Cc1ccccc1
Properties
Complexity:
345
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
245.105g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
245.278g/mol
Monoisotopic Mass:
245.105g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
57.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
1
XLogP3:
1.9
Literature
| Title | Journal |
|---|---|
| Reverse turn induced pi-facial selectivity during polyaniline-supported cobalt(II) salen catalyzed aerobic epoxidation of N-cinnamoyl L-proline derived peptides. | The Journal of organic chemistry 20030307 |
| Practical enantioselective synthesis of endothelin antagonist S-1255 by dynamic resolution of 4-methoxychromene-3-carboxylic acid intermediate. | The Journal of organic chemistry 20021101 |
| Stereoselective synthesis of beta-substituted phenylalanine-beta-phenylisoserine-derived tripeptides using N-cinnamoyl-L-proline as template: synthesis of structural analogues of HIV protease inhibitors. | The Journal of organic chemistry 20021101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.