200,000+ products from a single source!
sales@angenechem.com
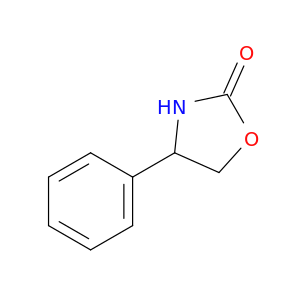
7480-32-2 | 4-Phenyloxazolidin-2-one
CAS No: 7480-32-2 Catalog No: AG005GY7 MDL No:MFCD00601044
Product Description
Catalog Number:
AG005GY7
Chemical Name:
4-Phenyloxazolidin-2-one
CAS Number:
7480-32-2
Molecular Formula:
C9H9NO2
Molecular Weight:
163.1733
MDL Number:
MFCD00601044
IUPAC Name:
4-phenyl-1,3-oxazolidin-2-one
InChI:
InChI=1S/C9H9NO2/c11-9-10-8(6-12-9)7-4-2-1-3-5-7/h1-5,8H,6H2,(H,10,11)
InChI Key:
QDMNNMIOWVJVLY-UHFFFAOYSA-N
SMILES:
O=C1OCC(N1)c1ccccc1
NSC Number:
409571
Properties
Complexity:
175
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
163.063g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
163.176g/mol
Monoisotopic Mass:
163.063g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
38.3A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
1.2
Literature
| Title | Journal |
|---|---|
| Stereoselectivities and regioselectivities of (4 + 3) cycloadditions between allenamide-derived chiral oxazolidinone-stabilized oxyallyls and furans: experiment and theory. | Journal of the American Chemical Society 20110914 |
| Vibrational spectra and molecular structure of chiral and racemic 4-phenyl-1,3-oxazolidin-2-one by density functional theory and ab initio Hartree-Fock calculations. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20060601 |
| Virtually complete control of simple and face diastereoselectivity in the Michael addition reactions between achiral equivalents of a nucleophilic glycine and (S)- or (R)-3-(E-enoyl)-4-phenyl-1,3-oxazolidin-2-ones: practical method for preparation of beta-substituted pyroglutamic acids and prolines. | The Journal of organic chemistry 20040723 |
Related Products
© 2019 Angene International Limited. All rights Reserved.