200,000+ products from a single source!
sales@angenechem.com
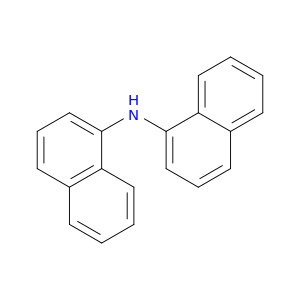
737-89-3 | 1,1-Dinaphthylamine , 98%
CAS No: 737-89-3 Catalog No: AG003D8S MDL No:MFCD00417046
Product Description
Catalog Number:
AG003D8S
Chemical Name:
1,1-Dinaphthylamine , 98%
CAS Number:
737-89-3
Molecular Formula:
C20H15N
Molecular Weight:
269.3398
MDL Number:
MFCD00417046
IUPAC Name:
N-naphthalen-1-ylnaphthalen-1-amine
InChI:
InChI=1S/C20H15N/c1-3-11-17-15(7-1)9-5-13-19(17)21-20-14-6-10-16-8-2-4-12-18(16)20/h1-14,21H
InChI Key:
VMVGVGMRBKYIGN-UHFFFAOYSA-N
SMILES:
c1ccc2c(c1)c(ccc2)Nc1cccc2c1cccc2
NSC Number:
12964
Properties
Complexity:
307
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
269.12g/mol
Formal Charge:
0
Heavy Atom Count:
21
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
269.347g/mol
Monoisotopic Mass:
269.12g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
12A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
6
Literature
| Title | Journal |
|---|---|
| Enantioselective intramolecular hydroamination catalyzed by lanthanide ate complexes coordinated by N-substituted (R)-1,1'-binaphthyl-2,2'-diamido ligands. | The Journal of organic chemistry 20060317 |
| Lanthanide complexes coordinated by N-substituted (R)-1,1'-binaphthyl-2,2'-diamido ligands in the catalysis of enantioselective intramolecular hydroamination. | Chemistry (Weinheim an der Bergstrasse, Germany) 20050520 |
| Synthesis and biological activities of bisnaphthalimido polyamines derivatives: cytotoxicity, DNA binding, DNA damage and drug localization in breast cancer MCF 7 cells. | Biochemical pharmacology 20050101 |
| The biological activities of new polyamine derivatives as potential therapeutic agents. | Biochemical Society transactions 20030401 |
Related Products
© 2019 Angene International Limited. All rights Reserved.