200,000+ products from a single source!
sales@angenechem.com
Home > Boronic Acids > 73183-34-3
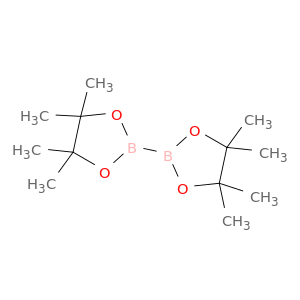
73183-34-3 | Bis(Pinacolato)Diboron
CAS No: 73183-34-3 Catalog No: AG0034J4 MDL No:MFCD00799570
Product Description
Catalog Number:
AG0034J4
Chemical Name:
Bis(Pinacolato)Diboron
CAS Number:
73183-34-3
Molecular Formula:
C12H24B2O4
Molecular Weight:
253.9386
MDL Number:
MFCD00799570
IUPAC Name:
4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolane
InChI:
InChI=1S/C12H24B2O4/c1-9(2)10(3,4)16-13(15-9)14-17-11(5,6)12(7,8)18-14/h1-8H3
InChI Key:
IPWKHHSGDUIRAH-UHFFFAOYSA-N
SMILES:
CC1(C)OB(OC1(C)C)B1OC(C(O1)(C)C)(C)C
EC Number:
615-925-0
UNII:
I906W26P4U
Properties
Complexity:
286
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
254.186g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
253.94g/mol
Monoisotopic Mass:
254.186g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
36.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Asymmetric 1,4-dihydroxylation of 1,3-dienes by catalytic enantioselective diboration. | Journal of the American Chemical Society 20090708 |
| Biaryl product formation from cross-coupling in palladium-catalyzed borylation of a Boc protected aminobromoquinoline compound. | Molecules (Basel, Switzerland) 20040227 |
| Suzuki-Miyaura homocoupling of naphthyl triflates using bis(pinacolato)diboron: approaches to the biaryl skeleton of crisamicin A. | Organic & biomolecular chemistry 20030621 |
| Palladium-catalyzed cross-coupling reaction of bis(pinacolato)diboron with 1-alkenyl halides or triflates: convenient synthesis of unsymmetrical 1,3-dienes via the borylation-coupling sequence. | Journal of the American Chemical Society 20020710 |
Related Products
© 2019 Angene International Limited. All rights Reserved.