200,000+ products from a single source!
sales@angenechem.com
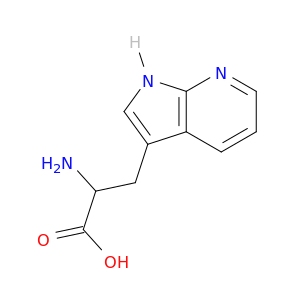
7303-50-6 | 1H-Pyrrolo[2,3-b]pyridine-3-propanoic acid, α-amino-
CAS No: 7303-50-6 Catalog No: AG0009D5 MDL No:MFCD00037930
Product Description
Catalog Number:
AG0009D5
Chemical Name:
1H-Pyrrolo[2,3-b]pyridine-3-propanoic acid, α-amino-
CAS Number:
7303-50-6
Molecular Formula:
C10H11N3O2
Molecular Weight:
205.2132
MDL Number:
MFCD00037930
IUPAC Name:
2-amino-3-(1H-pyrrolo[2,3-b]pyridin-3-yl)propanoic acid
InChI:
InChI=1S/C10H11N3O2/c11-8(10(14)15)4-6-5-13-9-7(6)2-1-3-12-9/h1-3,5,8H,4,11H2,(H,12,13)(H,14,15)
InChI Key:
SNLOIIPRZGMRAB-UHFFFAOYSA-N
SMILES:
NC(C(=O)O)Cc1c[nH]c2c1cccn2
NSC Number:
19495
Properties
Complexity:
247
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
205.085g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
205.217g/mol
Monoisotopic Mass:
205.085g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
92A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-2
Literature
| Title | Journal |
|---|---|
| Native state conformational heterogeneity of HP35 revealed by time-resolved FRET. | The journal of physical chemistry. B 20120906 |
| Azatryptophans as tools to study polarity requirements for folding of green fluorescent protein. | Journal of peptide science : an official publication of the European Peptide Society 20101001 |
| Non-natural amino acid fluorophores for one- and two-step fluorescence resonance energy transfer applications. | Analytical biochemistry 20100415 |
| Blue fluorescent amino acids as in vivo building blocks for proteins. | Chembiochem : a European journal of chemical biology 20100215 |
| Catalytic folding of the Cepsilon3 domain by its high affinity receptor. | FEBS letters 20060403 |
| Domain-specific incorporation of noninvasive optical probes into recombinant proteins. | Journal of the American Chemical Society 20041103 |
| Incorporation of the fluorescent amino acid 7-azatryptophan into the core domain 1-47 of hirudin as a probe of hirudin folding and thrombin recognition. | Protein science : a publication of the Protein Society 20040601 |
| In vivo properties of thiol inhibitors of the three vasopeptidases NEP, ACE and ECE are improved by introduction of a 7-azatryptophan in P2' position. | The journal of peptide research : official journal of the American Peptide Society 20040201 |
| Interaction of 7-azatryptophan and beta-(1-azulenyl)-alanine with a nitroxyl radical. | Advances in experimental medicine and biology 20030101 |
| A concerted structural transition in the plasminogen activator inhibitor-1 mechanism of inhibition. | Biochemistry 20021008 |
| Fluorescence resonance energy transfer between unnatural amino acids in a structurally modified dihydrofolate reductase. | Journal of the American Chemical Society 20020821 |
| Low temperature luminescence behaviours of 7-azatryptophan, 5-hydroxytryptophan and their chromophoric moieties. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20020701 |
| Crystal structure of the lytic transglycosylase from bacteriophage lambda in complex with hexa-N-acetylchitohexaose. | Biochemistry 20010515 |
Related Products
© 2019 Angene International Limited. All rights Reserved.