200,000+ products from a single source!
sales@angenechem.com
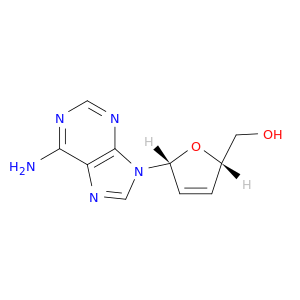
7057-48-9 | ((2S,5R)-5-(6-Amino-9H-purin-9-yl)-2,5-dihydrofuran-2-yl)methanol
CAS No: 7057-48-9 Catalog No: AG005HRC MDL No:MFCD00040965
Product Description
Catalog Number:
AG005HRC
Chemical Name:
((2S,5R)-5-(6-Amino-9H-purin-9-yl)-2,5-dihydrofuran-2-yl)methanol
CAS Number:
7057-48-9
Molecular Formula:
C10H11N5O2
Molecular Weight:
233.2266
MDL Number:
MFCD00040965
IUPAC Name:
[(2S,5R)-5-(6-aminopurin-9-yl)-2,5-dihydrofuran-2-yl]methanol
InChI:
InChI=1S/C10H11N5O2/c11-9-8-10(13-4-12-9)15(5-14-8)7-2-1-6(3-16)17-7/h1-2,4-7,16H,3H2,(H2,11,12,13)/t6-,7+/m0/s1
InChI Key:
JFUOUIPRAAGUGF-NKWVEPMBSA-N
SMILES:
OC[C@@H]1C=C[C@@H](O1)n1cnc2c1ncnc2N
UNII:
SK35877HI5
Properties
Complexity:
313
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
233.091g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
233.231g/mol
Monoisotopic Mass:
233.091g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
99.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.4
Literature
| Title | Journal |
|---|---|
| [Conformational capacity of 2',3'-didehydro-2',3'-dideoxyadenosine as a key to understanding its biological activity: results of quantum chemical modelling]. | Ukrains'kyi biokhimichnyi zhurnal (1999 ) 20110101 |
| Synthesis and antiviral evaluation of thieno[3,4-d]pyrimidine C-nucleoside analogues of 2',3'-dideoxy- and 2',3'-dideoxy-2',3'-didehydro-adenosine and -inosine. | Bioorganic & medicinal chemistry 20090315 |
| Effects of two novel nucleoside analogues on different hepatitis B virus promoters. | World journal of gastroenterology 20080328 |
| Efficacies of beta-L-D4A against Hepatitis B virus in 2.2.15 cells. | World journal of gastroenterology 20080227 |
| Inhibition of reverse transcriptase activity of hepatitis B virus polymerase by β-l-D4A-TP. | Acta virologica 20080101 |
| Effect and mechanism of beta-L-D4A on DNA polymerase alpha. | World journal of gastroenterology 20071214 |
| [Isosteric triphosphonate analogues of dNTP: synthesis and substrate properties toward various DNA polymerases]. | Bioorganicheskaia khimiia 20070101 |
| Synthesis and conformational analysis of new cyclobutane-fused nucleosides. | Organic letters 20060202 |
| [Synthesis of a novel L-nucleoside, beta-L-D4A and its inhibition on the replication of hepatitis B virus in vitro]. | Yao xue xue bao = Acta pharmaceutica Sinica 20050901 |
| Synthesis, structure-activity relationships, and drug resistance of beta-d-3'-fluoro-2',3'-unsaturated nucleosides as anti-HIV Agents. | Journal of medicinal chemistry 20040617 |
| Inhibition of hepatitis B virus by a novel L-nucleoside, beta-L-D4A and related analogues. | World journal of gastroenterology 20030815 |
| [Inhibition of the replication of hepatitis B virus in vitro by a novel nucleoside analogue (beta-L-D4A)]. | Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology 20030701 |
| [Effect and mechanism of beta-L-D4A (a novel nucleoside analog) against hepatitis B virus]. | Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology 20030501 |
| Synthesis and studies of 3'-C-trifluoromethyl nucleoside analogues bearing adenine or cytosine as the base. | Bioorganic & medicinal chemistry 20021001 |
| Stereoselective synthesis of a novel carbocyclic nucleoside. | The Journal of organic chemistry 20011102 |
| Activities of masked 2',3'-dideoxynucleoside monophosphate derivatives against human immunodeficiency virus in resting macrophages. | Antimicrobial agents and chemotherapy 20000101 |
| cycloSal-Pronucleotides of 2',3'-dideoxyadenosine and 2', 3'-dideoxy-2',3'-didehydroadenosine: synthesis and antiviral evaluation of a highly efficient nucleotide delivery system. | Journal of medicinal chemistry 19990506 |
| Conversion of 2',3'-dideoxyadenosine (ddA) and 2',3'-didehydro-2',3'-dideoxyadenosine (d4A) to their corresponding aryloxyphosphoramidate derivatives markedly potentiates their activity against human immunodeficiency virus and hepatitis B virus. | FEBS letters 19970630 |
| Potential prodrug derivatives of 2',3'-didehydro-2',3'-dideoxynucleosides. Preparations and antiviral activities. | Journal of medicinal chemistry 19920724 |
| Nucleic acid related compounds. 57. Synthesis, x-ray crystal structure, lipophilic partition properties, and antiretroviral activities of anomeric 3'-azido-2',3'-dideoxy-2,6-diaminopurine ribosides. | Journal of medicinal chemistry 19890801 |
| Comparative activity of 2',3'-saturated and unsaturated pyrimidine and purine nucleosides against human immunodeficiency virus type 1 in peripheral blood mononuclear cells. | Biochemical pharmacology 19881001 |
| Investigations on the anti-HIV activity of 2',3'-dideoxyadenosine analogues with modifications in either the pentose or purine moiety. Potent and selective anti-HIV activity of 2,6-diaminopurine 2',3'-dideoxyriboside. | Biochemical pharmacology 19880401 |
| Synthesis and anti-HIV activity of various 2'- and 3'-substituted 2',3'-dideoxyadenosines: a structure-activity analysis. | Journal of medicinal chemistry 19871101 |
| 3'-substituted 2',3'-dideoxynucleoside analogues as potential anti-HIV (HTLV-III/LAV) agents. | Journal of medicinal chemistry 19870801 |
| The anti-HTLV-III (anti-HIV) and cytotoxic activity of 2',3'-didehydro-2',3'-dideoxyribonucleosides: a comparison with their parental 2',3'-dideoxyribonucleosides. | Molecular pharmacology 19870701 |
| The 2',3'-dideoxyriboside of 2,6-diaminopurine selectively inhibits human immunodeficiency virus (HIV) replication in vitro. | Biochemical and biophysical research communications 19870529 |
| Both 2',3'-dideoxythymidine and its 2',3'-unsaturated derivative (2',3'-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. | Biochemical and biophysical research communications 19870115 |
Related Products
© 2019 Angene International Limited. All rights Reserved.