200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 69239-40-3
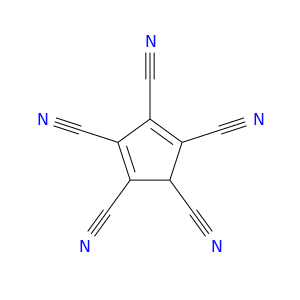
69239-40-3 | 1,3-Cyclopentadiene-1,2,3,4,5-pentacarbonitrile
CAS No: 69239-40-3 Catalog No: AG005K8X MDL No:
Product Description
Catalog Number:
AG005K8X
Chemical Name:
1,3-Cyclopentadiene-1,2,3,4,5-pentacarbonitrile
CAS Number:
69239-40-3
Molecular Formula:
C10HN5
Molecular Weight:
191.1484
IUPAC Name:
cyclopenta-1,3-diene-1,2,3,4,5-pentacarbonitrile
InChI:
InChI=1S/C10HN5/c11-1-6-7(2-12)9(4-14)10(5-15)8(6)3-13/h6H
InChI Key:
SZRONZXSOSCLOK-UHFFFAOYSA-N
SMILES:
N#CC1C(=C(C(=C1C#N)C#N)C#N)C#N
Properties
Complexity:
591
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
191.023g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
191.153g/mol
Monoisotopic Mass:
191.023g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
119A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.9
Literature
| Title | Journal |
|---|---|
| Group 11 complexes containing the [C5(CN)5]- ligand; 'coordination-analogues' of molecular organometallic systems. | Dalton transactions (Cambridge, England : 2003) 20120521 |
| Transition metal complexes of the pentacyanocyclopentadienide anion. | Chemical communications (Cambridge, England) 20110928 |
| Assembly of the first fullerene-type metal-organic frameworks using a planar five-fold coordination node. | Angewandte Chemie (International ed. in English) 20110829 |
| A simple approach to coordination compounds of the pentacyanocyclopentadienide anion. | Chemistry (Weinheim an der Bergstrasse, Germany) 20101210 |
| In search of ultrastrong Brønsted neutral organic superacids: a DFT study on some cyclopentadiene derivatives. | Chemistry (Weinheim an der Bergstrasse, Germany) 20041105 |
| Exploration of the pentacyano-cyclo-pentadienide ion, C(5)(CN)(5)(-), as a weakly coordinating anion and potential superacid conjugate base. Silylation and protonation. | Chemical communications (Cambridge, England) 20040321 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







