200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 68573-23-9
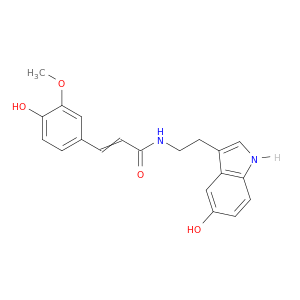
68573-23-9 | N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-3-(4-hydroxy-3-methoxy-phenyl)prop-2-enamide
CAS No: 68573-23-9 Catalog No: AG005PB2 MDL No:
Product Description
Catalog Number:
AG005PB2
Chemical Name:
N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-3-(4-hydroxy-3-methoxy-phenyl)prop-2-enamide
CAS Number:
68573-23-9
Molecular Formula:
C20H20N2O4
Molecular Weight:
352.3838
IUPAC Name:
(E)-N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-3-(4-hydroxy-3-methoxyphenyl)prop-2-enamide
InChI:
InChI=1S/C20H20N2O4/c1-26-19-10-13(2-6-18(19)24)3-7-20(25)21-9-8-14-12-22-17-5-4-15(23)11-16(14)17/h2-7,10-12,22-24H,8-9H2,1H3,(H,21,25)/b7-3+
InChI Key:
WGHKJYWENWLOMY-XVNBXDOJSA-N
SMILES:
COc1cc(C=CC(=O)NCCc2c[nH]c3c2cc(O)cc3)ccc1O
UNII:
2PP8322487
NSC Number:
369502
Properties
Complexity:
498
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
1
Exact Mass:
352.142g/mol
Formal Charge:
0
Heavy Atom Count:
26
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
352.39g/mol
Monoisotopic Mass:
352.142g/mol
Rotatable Bond Count:
6
Topological Polar Surface Area:
94.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3
Literature
| Title | Journal |
|---|---|
| N-Caffeoyl serotonin as selective COX-2 inhibitor. | Bioorganic & medicinal chemistry letters 20120401 |
| Synthesis, biological activities and bioavailability of moschamine, a safflomide-type phenylpropenoic acid amide found in Centaurea cyanus. | Natural product research 20120101 |
| Effect of N-(p-coumaroyl)serotonin and N-feruloylserotonin, major anti-atherogenic polyphenols in safflower seed, on vasodilation, proliferation and migration of vascular smooth muscle cells. | Molecular nutrition & food research 20111001 |
| Methanol elicits the biosynthesis of 4-coumaroylserotonin and feruloylserotonin in rice seedlings. | Plant signaling & behavior 20110601 |
| Synthesis and structure-activity relationships of phenylpropanoid amides of serotonin on tyrosinase inhibition. | Bioorganic & medicinal chemistry letters 20110401 |
| Naturally appearing N-feruloylserotonin isomers suppress oxidative burst of human neutrophils at the protein kinase C level. | Pharmacological reports : PR 20110101 |
| Protective effect of serotonin derivatives on glucose-induced damage in PC12 rat pheochromocytoma cells. | The British journal of nutrition 20100101 |
| Suppression of oxidative burst in human neutrophils with the naturally occurring serotonin derivative isomer from Leuzea carthamoides. | Neuro endocrinology letters 20100101 |
| Safflower seed polyphenols (N-(p-coumaroyl)serotonin and N-feruloylserotonin) ameliorate atherosclerosis and distensibility of the aortic wall in Kurosawa and Kusanagi-hypercholesterolemic (KHC) rabbits. | Hypertension research : official journal of the Japanese Society of Hypertension 20091101 |
| N-[(Dihydroxyphenyl)acyl]serotonins as potent inhibitors of tyrosinase from mouse and human melanoma cells. | Bioorganic & medicinal chemistry letters 20090801 |
| Inhibitory effect of serotonin derivatives on high glucose-induced adhesion and migration of monocytes on human aortic endothelial cells. | The British journal of nutrition 20090701 |
| The selective effect of N-feruloylserotonins isolated from Leuzea carthamoides on nociception and anxiety in rats. | Journal of ethnopharmacology 20070613 |
| Serotonin derivatives, major safflower (Carthamus tinctorius L.) seed antioxidants, inhibit low-density lipoprotein (LDL) oxidation and atherosclerosis in apolipoprotein E-deficient mice. | Journal of agricultural and food chemistry 20060712 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.