200,000+ products from a single source!
sales@angenechem.com
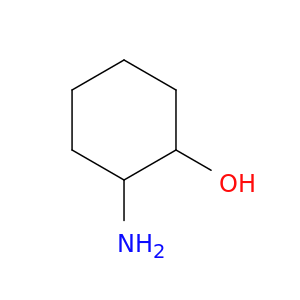
6850-38-0 | 2-Aminocyclohexanol
CAS No: 6850-38-0 Catalog No: AG003346 MDL No:MFCD00191368
Product Description
Catalog Number:
AG003346
Chemical Name:
2-Aminocyclohexanol
CAS Number:
6850-38-0
Molecular Formula:
C6H13NO
Molecular Weight:
115.1735
MDL Number:
MFCD00191368
IUPAC Name:
2-aminocyclohexan-1-ol
InChI:
InChI=1S/C6H13NO/c7-5-3-1-2-4-6(5)8/h5-6,8H,1-4,7H2
InChI Key:
PQMCFTMVQORYJC-UHFFFAOYSA-N
SMILES:
NC1CCCCC1O
Properties
Complexity:
74.9
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
115.1g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
115.176g/mol
Monoisotopic Mass:
115.1g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
46.2A^2
Undefined Atom Stereocenter Count:
2
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.1
Literature
| Title | Journal |
|---|---|
| Estimating the lipophilicity of a number of 2-amino-1-cyclohexanol derivatives exhibiting anticonvulsant activity. | Biomedical chromatography : BMC 20090501 |
| trans-2-Aminocyclohexanol as a pH-sensitive conformational switch in lipid amphiphiles. | Chemical communications (Cambridge, England) 20081021 |
| Leishmanicidal and trypanocidal activities of 2-aminocyclohexanol and 1,2-cyclohexanediamine derivatives. | Bioorganic & medicinal chemistry letters 20080101 |
| A novel NADP+-dependent L-1-amino-2-propanol dehydrogenase from Rhodococcus erythropolis MAK154: a promising enzyme for the production of double chiral aminoalcohols. | Letters in applied microbiology 20061001 |
| Amino alcohols as ligands for nickel-catalyzed suzuki reactions of unactivated alkyl halides, including secondary alkyl chlorides, with arylboronic acids. | Journal of the American Chemical Society 20060426 |
| Resolution of racemic 2-aminocyclohexanol derivatives and their application as ligands in asymmetric catalysis. | The Journal of organic chemistry 20060317 |
| Copper(II) complexes of aminocarbohydrate beta-ketoenaminic ligands: efficient catalysts in catechol oxidation. | Chemistry (Weinheim an der Bergstrasse, Germany) 20010518 |
Related Products
© 2019 Angene International Limited. All rights Reserved.