200,000+ products from a single source!
sales@angenechem.com
Home > Fluorides > 680611-86-3
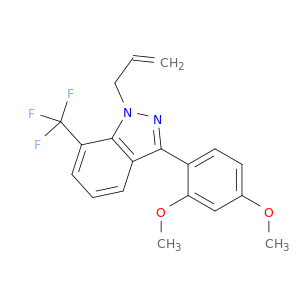
680611-86-3 | 1H-Indazole, 3-(2,4-dimethoxyphenyl)-1-(2-propenyl)-7-(trifluoromethyl)-
CAS No: 680611-86-3 Catalog No: AG006G3J MDL No:
Product Description
Catalog Number:
AG006G3J
Chemical Name:
1H-Indazole, 3-(2,4-dimethoxyphenyl)-1-(2-propenyl)-7-(trifluoromethyl)-
CAS Number:
680611-86-3
Molecular Formula:
C19H17F3N2O2
Molecular Weight:
362.3457
IUPAC Name:
3-(2,4-dimethoxyphenyl)-1-prop-2-enyl-7-(trifluoromethyl)indazole
InChI:
InChI=1S/C19H17F3N2O2/c1-4-10-24-18-14(6-5-7-15(18)19(20,21)22)17(23-24)13-9-8-12(25-2)11-16(13)26-3/h4-9,11H,1,10H2,2-3H3
InChI Key:
BMIOASGFHBRKJL-UHFFFAOYSA-N
SMILES:
C=CCn1nc(c2c1c(ccc2)C(F)(F)F)c1ccc(cc1OC)OC
Properties
Complexity:
488
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
362.124g/mol
Formal Charge:
0
Heavy Atom Count:
26
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
362.352g/mol
Monoisotopic Mass:
362.124g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
36.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.8
Literature
| Title | Journal |
|---|---|
| Editor's Highlight: Ah Receptor Activation Potentiates Neutrophil Chemoattractant (C-X-C Motif) Ligand 5 Expression in Keratinocytes and Skin. | Toxicological sciences : an official journal of the Society of Toxicology 20171101 |
| Differential regulation of Th17 and T regulatory cell differentiation by aryl hydrocarbon receptor dependent xenobiotic response element dependent and independent pathways. | Toxicological sciences : an official journal of the Society of Toxicology 20150601 |
| Selective aryl hydrocarbon receptor modulator-mediated repression of CD55 expression induced by cytokine exposure. | The Journal of pharmacology and experimental therapeutics 20120801 |
| Identification of a high-affinity ligand that exhibits complete aryl hydrocarbon receptor antagonism. | The Journal of pharmacology and experimental therapeutics 20110701 |
| Development of a selective modulator of aryl hydrocarbon (Ah) receptor activity that exhibits anti-inflammatory properties. | Chemical research in toxicology 20100517 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.